Metabolism: Regulation & Integration
🔹Let’s look at the core pathways, & whether they are anabolic, catabolic, or amphibolic (both)🔹
What is Metabolism?
Metabolism refers to all chemical reactions in the body that:
a. Maintain life and homeostasis
b. Provide energy (ATP) &
c. Support growth, repair, reproduction, and survival
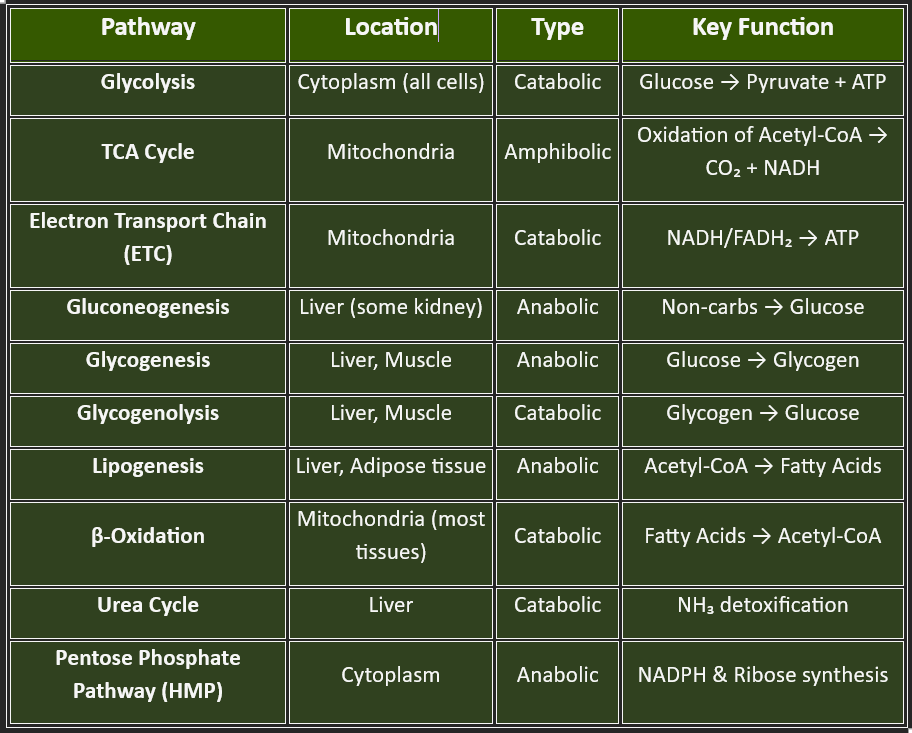
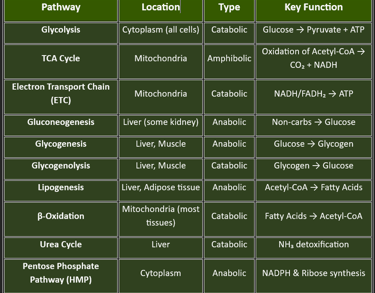
🔹 I. MAJOR METABOLIC PATHWAYS🔹
Let me break down this topic into three main parts for easy understanding:
I. MAJOR METABOLIC PATHWAYS
II. CONTROL MECHANISMS OF METABOLIC PATHWAYS
III. METABOLIC PROFILE OF ORGANS
II. CONTROL MECHANISMS OF METABOLIC PATHWAY
Why Control Is Necessary?
Prevents waste of energy (anabolism and catabolism don’t run simultaneously at full speed).
Maintains homeostasis (constant internal environment).
Allows adaptation to different nutritional and physiological states (fasting, exercise, stress).
Coordinates between different organs (like the liver, muscle, and brain).
Metabolism is like a well-organized city: roads (pathways) carry traffic (metabolites), signals (enzymes and hormones) direct the flow, and checkpoints prevent chaos. If every road were open with no signals, there would be gridlock. Similarly, without control mechanisms, metabolism would collapse.
✨ In short: Control mechanisms of metabolic pathways are like the traffic rules of life — ensuring smooth flow, preventing accidents, and allowing the body to adapt to ever-changing needs.
👉Medical students must understand how the body regulates these pathways — because disruptions lead to diseases like diabetes, cancer, or inborn errors of metabolism.
Major Control Mechanisms
1. Allosteric Regulation
2. Covalent Modification
3. Genetic Control (Enzyme Induction & Repression)
4. Compartmentalization
5. Feedback Inhibition
6. Energy Charge of the Cell (ATP/ADP/AMP ratio)
7. Hormonal Regulation
1. Allosteric Regulation
Definition: Enzymes are switched on/off by molecules binding at sites other than the active site.
Example: Phosphofructokinase-1 (PFK-1) in glycolysis is activated by AMP and inhibited by ATP & citrate.
Clinical relevance: Explains why glycolysis speeds up when energy is low.
7. Hormonal Regulation
Insulin, glucagon, cortisol, and thyroid hormones act as “long-distance messengers.”
Example:
Insulin promotes glucose uptake & storage.
Glucagon mobilizes glucose in fasting.
6. Energy Charge of the Cell (ATP/ADP/AMP ratio)
Acts as a global regulator.
High ATP → slows catabolism, promotes anabolism.
High AMP → activates catabolism (ATP-producing pathways).
4. Compartmentalization
Definition: Pathways are separated into different cellular compartments to avoid conflict.
Example:
Fatty acid oxidation occurs in mitochondria.
Fatty acid synthesis occurs in the cytoplasm.
Prevents futile cycles.
5. Feedback Inhibition
Definition: The End product of a pathway inhibits the first committed enzyme.
Example: Cholesterol synthesis is regulated by cholesterol-inhibiting HMG-CoA reductase.
Clinical relevance: Target for statin drugs in hypercholesterolemia.
2. Covalent Modification
Definition: Enzymes are activated or inactivated by reversible covalent changes (mainly phosphorylation/dephosphorylation).
Example: Glycogen phosphorylase (breaks glycogen) is activated by phosphorylation.
Hormonal link: Mediated by insulin, glucagon, and epinephrine.
3. Genetic Control (Enzyme Induction & Repression)
Definition: Regulation at the DNA/RNA level; cells increase or decrease enzyme synthesis.
Example: Liver enzyme levels (e.g., cytochrome P450) rise with certain drugs.
Clinical relevance: Basis for drug tolerance and cancer gene regulation.
🩺 CLINICAL RELEVANCE
👉Control mechanisms are not just theory — they explain why drugs work, why diseases happen, and how treatments are designed.
👉Linking control mechanisms of metabolic pathways to clinical relevance is what makes biochemistry come alive.
1. Feedback Inhibition (End-Product Inhibition)
Mechanism: The end product of a pathway inhibits an enzyme at the beginning.
Clinical Relevance:
Cholesterol metabolism → Cholesterol inhibits HMG-CoA reductase.
This is the basis of statin drugs used in treating high cholesterol.
Purine synthesis → Excess purines inhibit key enzymes.
Dysregulation may lead to gout due to uric acid buildup.
4. Hormonal Regulation
Mechanism: Hormones like insulin, glucagon, and cortisol regulate metabolism.
Clinical Relevance:
Diabetes mellitus → defective insulin signaling leads to uncontrolled gluconeogenesis, lipolysis, and ketone body formation.
Cushing’s syndrome → excess cortisol alters protein and fat metabolism.
2. Compartmentalization
Mechanism: Pathways occur in different organelles.
Clinical Relevance:
Fatty acid synthesis (cytoplasm) vs oxidation (mitochondria).
Defects in transport systems (like carnitine deficiency) impaired oxidation → leading to muscle weakness, hypoglycemia.
Urea cycle (mitochondria + cytoplasm).
3. Allosteric Regulation
Mechanism: Enzymes are activated or inhibited by molecules binding at sites other than the active site.
Clinical Relevance:
Phosphofructokinase (PFK-1) in glycolysis is regulated by ATP, citrate, and AMP.
In cancer, dysregulated glycolysis (Warburg effect) occurs due to altered allosteric control.
👉 Summary
These control mechanisms aren’t just abstract biochemistry—they are the same switches and sensors your body uses every moment to survive.
When they go wrong, you see diseases like diabetes, obesity, heart failure, or even cancer.
And when doctors prescribe drugs like metformin, statins, or kinase inhibitors, they’re really just tapping into these natural metabolic control systems.
✨ How to remember some Tips-
Think of the body like a smart city:
Feedback inhibition = traffic signals to prevent congestion.
Compartmentalization = zoning of industries (different work in different areas).
Covalent modification = switches turning lights on/off instantly.
Genetic control = city planning documents (long-term regulation).
Energy charge = power supply meter deciding if factories should run or pause.
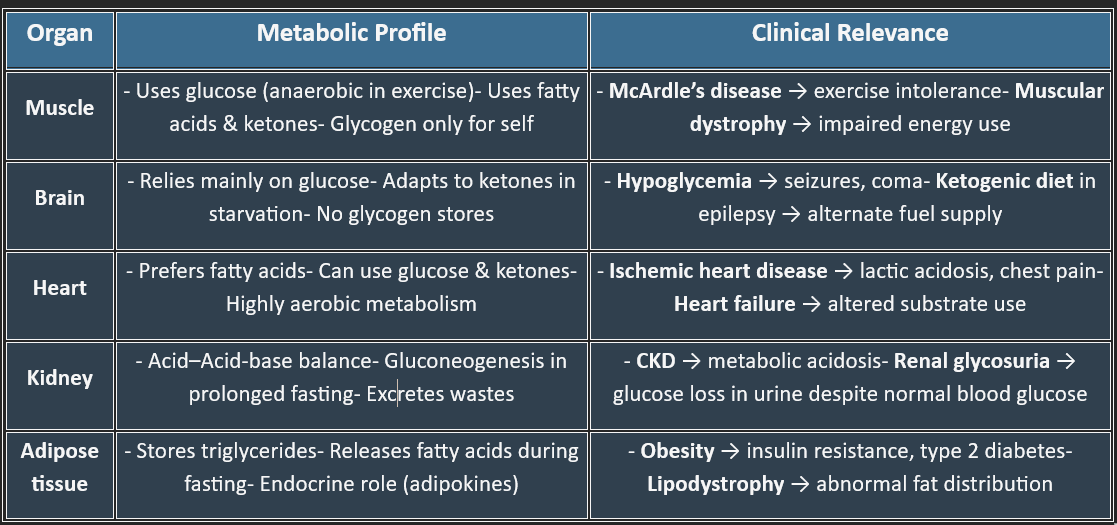
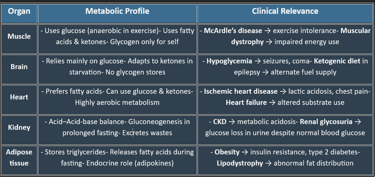
III. METABOLIC PROFILE OF ORGANS
Introduction
The human body is a complex biochemical factory where different organs exhibit specialized metabolic functions. Understanding the metabolic profile of organs is crucial for both medical and dental students, as it forms the foundation for comprehending physiological functions, pathological conditions, and clinical interventions. Each organ has a distinct metabolic role based on its energy demands and function, and any disruption in these pathways can lead to systemic disorders.
🔷 What is a Metabolic Profile? 🔷
The metabolic profile of an organ refers to its unique set of biochemical functions, fuel preferences, enzyme expression patterns, and metabolic roles within the body.
Each organ is biochemically specialized based on its structure, function, and energy demands.
1. Brain: The Glucose-Hungry Organ
2. Liver: The Metabolic Hub
3. Muscle: Powerhouse of Movement
4. Adipose Tissue: The Energy Reservoir
5. Kidney: The Filtration & Regulatory Organ
6. Heart: The High-Energy Pump
7. Erythrocytes – Anaerobic Survivors
8. Gastrointestinal Tract: The Digestive & Absorptive Machine
9. Pancreas: The Endocrine & Exocrine Gland
🩺 Clinical Relevance of the Metabolic Profile of Organs
The metabolic profile of organs (who does what, who consumes what, and who produces what) is not just a memory exercise — it has direct clinical relevance in medicine and dentistry.
Each organ has a distinct metabolic “personality.” When its preferred pathway is disturbed, specific diseases appear — this is why understanding metabolic profiles is crucial for diagnosis and treatment.
1. Liver – The Metabolic Hub
Profile:
Performs gluconeogenesis, glycogen storage, the urea cycle, cholesterol synthesis, and drug detoxification.
Clinical Relevance:
Liver failure/cirrhosis → hypoglycemia (no gluconeogenesis), hyperammonemia (failed urea cycle), coagulopathy (no clotting factors).
Drug toxicity (e.g., paracetamol overdose) due to impaired detox.
4. Heart – The Fatty Acid Burner
Profile:
Prefers fatty acids; can use glucose and ketones if needed.
Clinical Relevance:
Ischemic heart disease → blocked O₂ supply prevents β-oxidation → shifts to anaerobic glycolysis → lactic acidosis & chest pain.
Heart failure alters substrate use, which is targeted in new metabolic drugs.
2. Muscle – The Power Consumer
Profile:
Uses glucose (anaerobic during exercise), fatty acids, and ketone bodies.
Stores glycogen but only for its own use.
Clinical Relevance:
McArdle’s disease (glycogen storage disorder) → exercise intolerance, muscle cramps.
Muscular dystrophy → altered energy utilization.
5. Kidney – The Filter and Gluconeogenic Backup
Profile:
Maintains acid-base balance, excretes wastes, and performs gluconeogenesis in prolonged fasting.
Clinical Relevance:
Chronic kidney disease → metabolic acidosis (failure of H⁺ excretion, bicarbonate regeneration).
Renal glycosuria → defective glucose reabsorption despite normal blood glucose.
3. Brain – The Glucose Addict
Profile:
Relies mainly on glucose; in starvation → adapts to ketone bodies.
No glycogen storage.
Clinical Relevance:
Hypoglycemia → confusion, seizures, coma (because the brain cannot store glucose).
Ketogenic diets used in epilepsy → provide an alternative fuel.
6. Adipose Tissue – The Energy Storehouse
Profile:
Stores triglycerides, releases free fatty acids when needed.
Clinical Relevance:
Obesity → excessive fat storage linked to insulin resistance, type 2 diabetes.
Lipodystrophy → abnormal fat distribution → metabolic syndrome.
✅ Summary Table
BLOG
Join us to explore medical biochemistry intricacies.
WRITE TO US
© 2024. All rights reserved.
