Carbohydrate Metabolism
Fueling the Biochemical Symphony of Life
“Glucose is more than sugar—it’s the universal currency of energy, the lifeblood of neurons, and a marker of health and disease.”
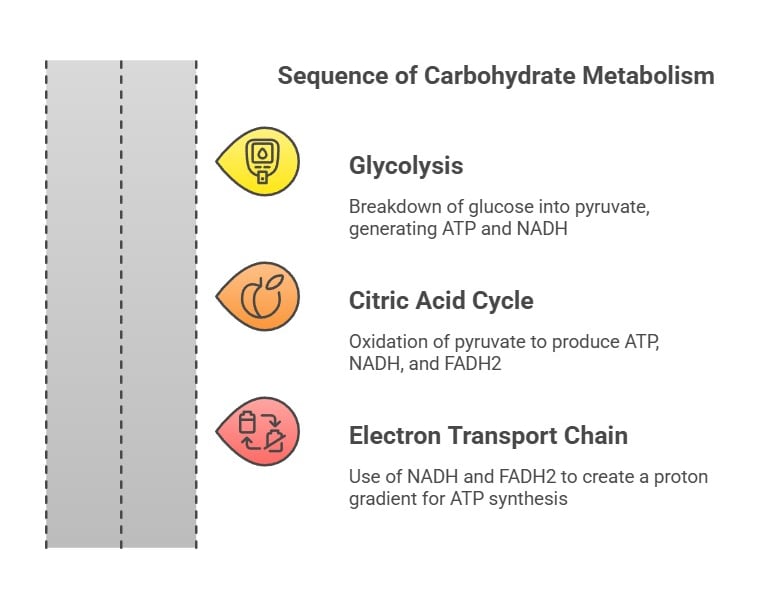
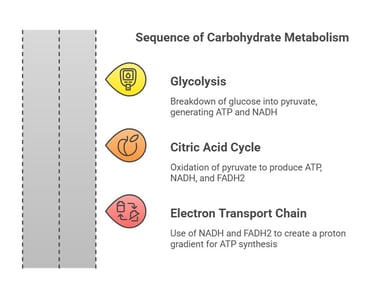
1. Catabolism: (The breakdown of saccharides)
Glycolysis: This is the breakdown of glucose into pyruvate, generating ATP and NADH.
Citric Acid Cycle (Krebs Cycle): Here, pyruvate is oxidized, producing ATP, NADH, and FADH2, which are used in the electron transport chain.
Electron Transport Chain: NADH and FADH2 donate electrons, creating a proton gradient that drives ATP synthesis.
2. Anabolism (The synthesis of saccharides)
Gluconeogenesis: The generation of glucose from non-carbohydrate sources like amino acids.
Glycogenesis: The process of glycogen synthesis, storing glucose in the liver and muscles.
Pentose Phosphate Pathway: Generates NADPH and ribose-5-phosphate for nucleotide synthesis.
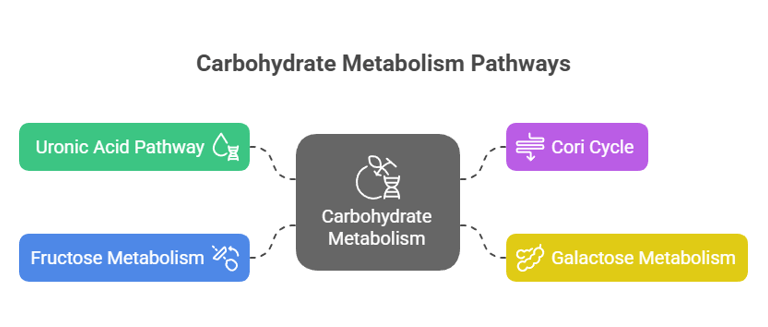
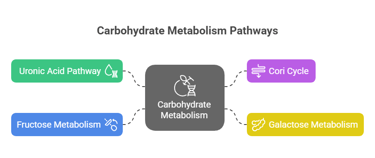
3. Other Metabolic Pathways
Uronic Acid Pathway: Converts glucose to glucuronic acid, crucial for detoxifying drugs and forming vitamin C in some animals.
Cori Cycle: Lactate produced in muscles during anaerobic respiration is transported to the liver, converted to glucose, and sent back to muscles.
Galactose Metabolism
Fructose Metabolism
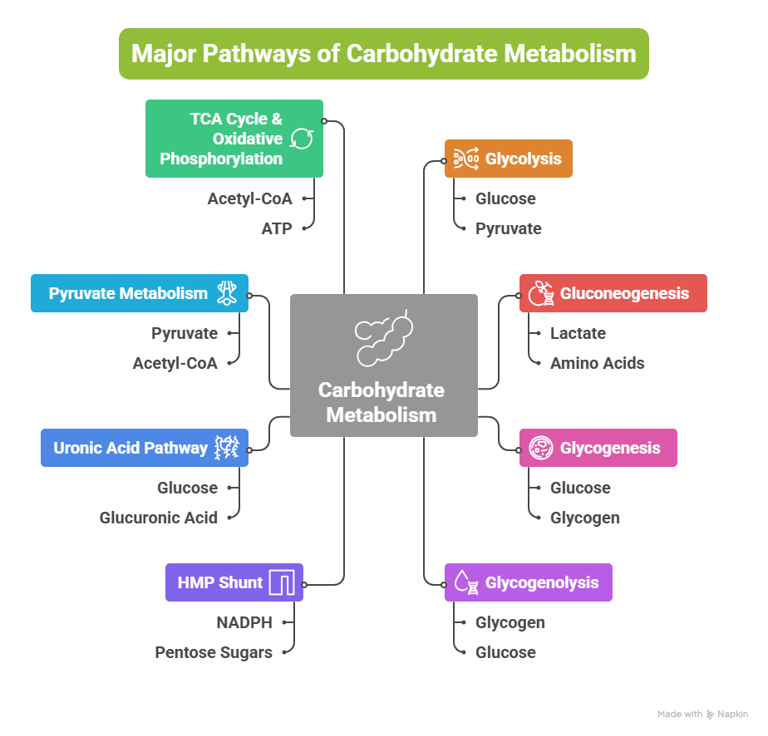
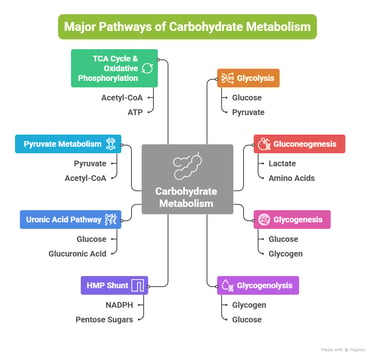
Overview: Major Pathways of Carbohydrate Metabolism
Introduction: Why Carbohydrate Metabolism Matters in Medicine
Carbohydrates, primarily glucose, serve as:
The main energy source for all tissues
The sole fuel for RBCs, the brain (under normal conditions), and the renal medulla
A precursor for amino acids, nucleotides, and fatty acids
Conclusion
Carbohydrate metabolism is a fundamental biochemistry that integrates:
Energy generation and storage
Stress and fasting physiology
Metabolic disorders, diabetes, and inborn errors
“To master carbohydrate metabolism is to understand the biochemical language of life’s most immediate energy needs.”
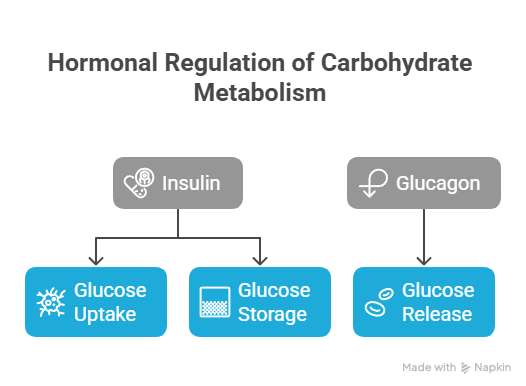
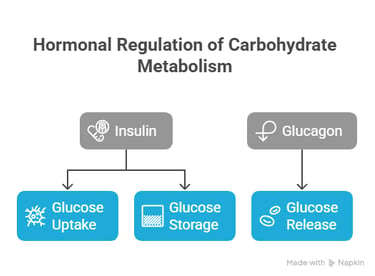
Hormonal Regulation of Carbohydrate Metabolism
Insulin and glucagon are primary hormones regulating carbohydrate metabolism.
Insulin promotes glucose uptake and storage,
Glucagon stimulates glucose release into the bloodstream.
Biomedical Significance
Proper regulation of carbohydrate metabolism is crucial for maintaining energy balance and blood glucose levels. Dysregulation can lead to conditions like diabetes mellitus, hypoglycemia, and metabolic syndrome.
Understanding these pathways provides insight into how the body utilizes and regulates energy, which is essential for developing treatments for metabolic disorders.
Carbohydrate metabolism involves a network of interconnected pathways:
Glycolysis
Gluconeogenesis
Glycogenesis
Glycogenolysis
Hexose Monophosphate (HMP) Shunt
Uronic Acid Pathway
Pyruvate Metabolism
TCA Cycle & Oxidative Phosphorylation
Other metabolic pathways include: (Fructose, Galactose, Lactose, Cori cycle, BPG Cycle)
Carbohydrates, primarily glucose, serve as:
The main energy source for all tissues
The sole fuel for RBCs, the brain (under normal conditions), and the renal medulla
A precursor for amino acids, nucleotides, and fatty acids
⚕️As a medical student, understanding carbohydrate metabolism helps decode:
Diabetes mellitus, hypoglycemia, lactic acidosis
Glycogen storage diseases
Clinical tests like HbA1c, GTT, C-peptide, and RBS
Why Carbohydrate Metabolism Matters in Medicine
✍️ Quick Notes for Understanding:
Catabolic: Breaks molecules to release energy (e.g., glycolysis, glycogenolysis).
Anabolic: Builds complex molecules using energy (e.g., gluconeogenesis, glycogenesis).
Amphibolic: Functions in both anabolism and catabolism (e.g., TCA cycle).
HMP Shunt provides reducing power (NADPH) for anabolic reactions & ribose sugars for nucleotide synthesis.
The Uronic acid pathway helps in detoxification by forming conjugates with bilirubin, drugs, etc.
Carbohydrate metabolism is a fundamental biochemistry that integrates:
1. Energy generation and storage
2. Stress and fasting physiology
3. Metabolic disorders, diabetes, and inborn errors
“To master carbohydrate metabolism is to understand the biochemical language of life’s most immediate energy needs.”
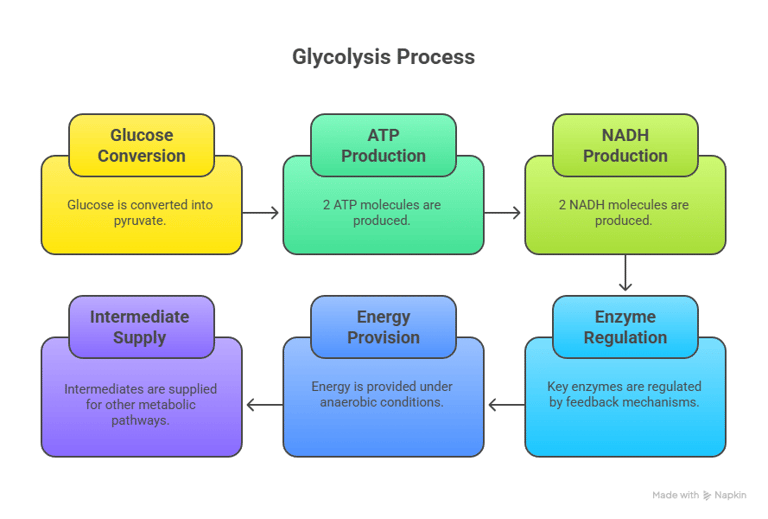
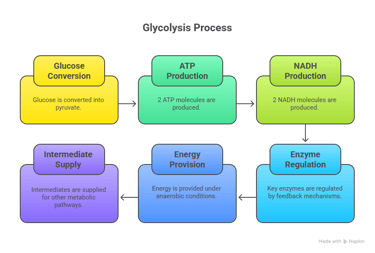
⚡ 1. Glycolysis – The Central Energy Pathway
Location: Cytoplasm of all cells/End-product: Pyruvate (aerobic) or Lactate (anaerobic)
Net Reaction: Glucose + 2 ADP + 2 Pi + 2 NAD⁺ → 2 Pyruvate + 2 ATP + 2 NADH + 2 H⁺
Key Regulatory Enzymes:
Hexokinase / Glucokinase (liver-specific)
Phosphofructokinase-1 (PFK-1) – Rate-limiting step
Pyruvate kinase
Clinical Relevance:
Pyruvate kinase deficiency → Hemolytic anemia
Cancer cells show increased glycolysis (Warburg effect)
Lactic acidosis occurs during hypoxia/sepsis due to anaerobic glycolysis
Introduction to Glycolysis
Definition: Glycolysis is the metabolic pathway that converts glucose into pyruvate, producing ATP and NADH.
Location: Cytoplasm of all cells.
Importance: First step in glucose catabolism.
🧭Glycolysis Pathway Steps (I)
1. Energy Investment Phase (Steps 1–5):
Glucose phosphorylation → Fructose-1,6-bisphosphate formation.
Enzymes: Hexokinase, Phosphofructokinase-1 (PFK-1).
ATP consumed: 2 molecules.
Note: Now, 2 molecules of G3P proceed through the remaining steps.
🧭Glycolysis Pathway Steps (II)
2. Energy Payoff Phase (Steps 6–10):
Glyceraldehyde-3-phosphate oxidation → Pyruvate formation.
Enzymes: Glyceraldehyde-3-phosphate dehydrogenase, Pyruvate kinase.
ATP produced: 4 molecules.
NADH produced: 2 molecules.
2. Gluconeogenesis – Glucose from Non-Carbs
Bypass Enzymes:
Pyruvate carboxylase
PEP carboxykinase
Fructose-1,6-bisphosphatase
Glucose-6-phosphatas
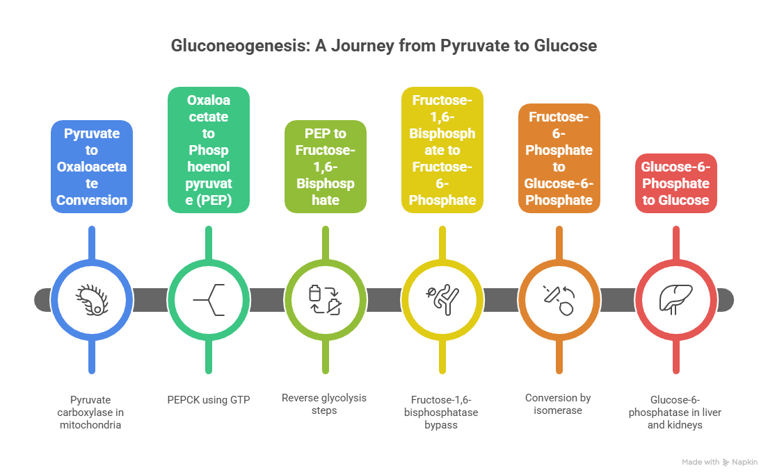
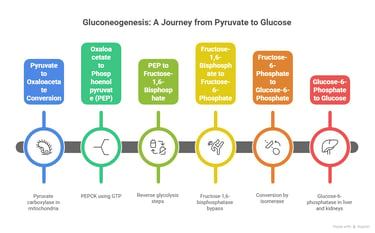
Gluconeogenesis involves multiple steps that occur in both the cytoplasm and mitochondria of the liver
Location: Liver (mostly), kidney (partially)/ Precursors: Lactate, alanine, glycerol
Clinical Relevance:
Essential during fasting, stress, and starvation
Defects → hypoglycemia, lactic acidosis, hyperuricemia
Von Gierke’s disease: Deficiency of G6Pase → hepatomegaly, fasting hypoglycemia
Gluconeogenesis is the synthesis of glucose from non-carbohydrate precursors. It is essentially the reverse of glycolysis, but with a few key regulatory steps to bypass the irreversible reactions in glycolysis.
This process primarily occurs in the liver (and to a lesser extent in the kidneys) during times of fasting, starvation, or intense exercise when glucose reserves from glycogen are depleted.
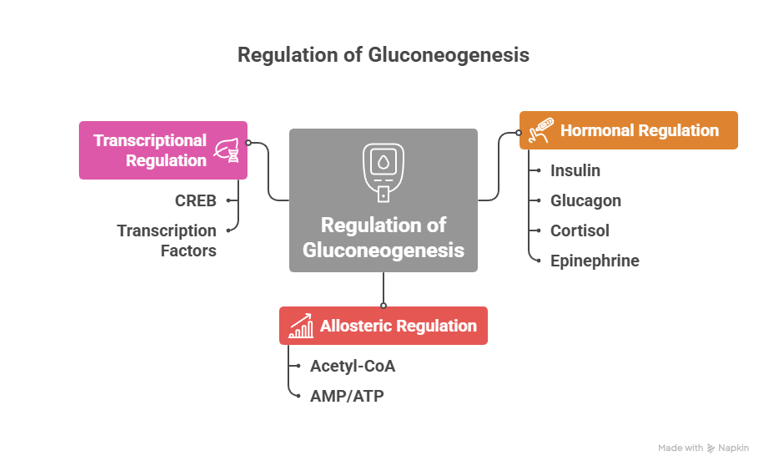
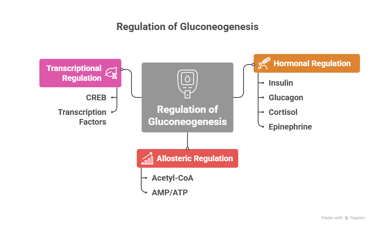
Regulation of Gluconeogenesis
The regulation of gluconeogenesis ensures that glucose is produced only when needed, and the process is tightly controlled:
1. Hormonal Regulation
· Insulin: Inhibits gluconeogenesis. When blood glucose levels are high (such as after eating), insulin levels increase and suppress the production of key gluconeogenic enzymes like phosphoenolpyruvate carboxykinase (PEPCK) and fructose-1,6-bisphosphatase.
· Glucagon: Stimulates gluconeogenesis. When blood glucose is low (e.g., during fasting), glucagon levels increase, activating transcription factors that enhance the expression of gluconeogenic enzymes.
· Cortisol: Stimulates gluconeogenesis, particularly during stress or prolonged fasting. It increases the availability of amino acids (precursors for gluconeogenesis) by promoting protein breakdown.
· Epinephrine: During stress or exercise, epinephrine promotes gluconeogenesis and glycogen breakdown to provide glucose for muscles and other tissues.
2. Allosteric Regulation
· Acetyl-CoA: High levels of acetyl-CoA stimulate gluconeogenesis by activating pyruvate carboxylase, the enzyme that converts pyruvate to oxaloacetate, initiating the gluconeogenic pathway.
· AMP/ATP: AMP (indicative of a low-energy state) inhibits gluconeogenesis, while ATP (indicative of a high-energy state) promotes it.
3. Transcriptional Regulation
· CREB (cAMP response element-binding protein) and other transcription factors regulate the expression of enzymes such as PEPCK and fructose-1,6-bisphosphatase in response to hormonal signals like glucagon and cortisol.
Why Does Our Body Need Gluconeogenesis?
Maintaining Blood Glucose Levels
Gluconeogenesis ensures the body maintains a constant supply of glucose, particularly during fasting, starvation, or prolonged exercise when glycogen stores are depleted.
It helps maintain blood glucose levels for tissues that rely on glucose for energy, particularly the brain and red blood cells.
Preventing Hypoglycemia
When glycogen stores are depleted (e.g., after a night of fasting), gluconeogenesis becomes the primary means of glucose production, preventing hypoglycemia (low blood glucose levels), which can impair brain function.
Sustaining Energy in Low-Carbohydrate Conditions
In conditions like low-carb diets or diabetes, the body still needs a way to produce glucose from other sources. Gluconeogenesis plays a crucial role in these situations.
Utilization of Non-Carbohydrate Sources
Gluconeogenesis allows the body to convert amino acids from muscle breakdown and glycerol from fat metabolism into glucose, preserving muscle mass and fat stores for energy during prolonged periods without food.
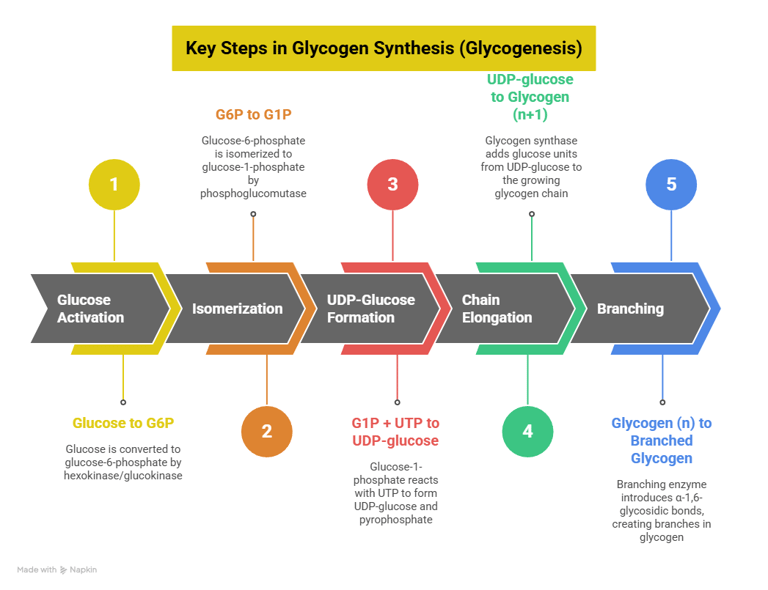
3. Glycogenesis – Storage of Glucose
📍 Location: Liver and muscle🔑 Key Enzyme: Glycogen synthase
🔁 Steps: Glucose → G6P → G1P → UDP-glucose → Glycogen
Clinical Relevance:
Regulated by insulin (activates) and glucagon (inhibits)
Deficiency → Andersen’s disease (branching enzyme defect)
Conclusion
Glycogenesis is the process of glucose storage in the form of glycogen and plays a central role in maintaining glucose homeostasis. It is tightly regulated by hormones like insulin, glucagon, and epinephrine to ensure glucose is stored when in excess and mobilized when needed. Dysregulation of glycogenesis can lead to metabolic disorders such as diabetes and glycogen storage diseases.
Clinical Significance of Glycogenesis
Glycogen Storage Diseases (GSDs):
Glycogenesis enzyme deficiencies can lead to glycogen storage diseases. These conditions impair the ability to synthesize glycogen properly, resulting in abnormal accumulation or depletion of glycogen in tissues.
For example, GSD Type 0 results from a deficiency in glycogen synthase, leading to hypoglycemia and poor glycogen storage.
Diabetes Mellitus:
In type 2 diabetes, there is often impaired insulin signaling, leading to impaired glycogenesis. This contributes to hyperglycemia and an inability to store excess glucose efficiently.
Starvation and Fasting:
In prolonged starvation or fasting, glycogenesis is inhibited by low insulin and high glucagon levels, while glycogenolysis (glycogen breakdown) is activated to maintain blood glucose levels.
Regulation of Glycogenesis
Glycogenesis is highly regulated by several mechanisms to ensure that glycogen is synthesized when the body has excess glucose (such as after meals) and not during fasting or energy-demanding conditions.
1. Hormonal Regulation
Insulin (Activation):
Insulin, released from the pancreas after eating, promotes glycogenesis by stimulating the enzymes involved in glycogen synthesis.
Insulin activates protein phosphatases, which dephosphorylate and activate glycogen synthase, the key enzyme in the synthesis of glycogen.
Insulin also inhibits glycogen phosphorylase, the enzyme responsible for breaking down glycogen.
Glucagon (Inhibition in Liver):
Glucagon, released when blood glucose levels are low (during fasting), inhibits glycogenesis in the liver by activating protein kinase A (PKA).
PKA phosphorylates and inactivates glycogen synthase, thus preventing glycogen synthesis.
Epinephrine (Inhibition in Muscle and Liver):
Epinephrine, released during stress or exercise, also activates PKA, inhibiting glycogen synthase and promoting glycogen breakdown, especially in muscles.
2. Allosteric Regulation
Glucose-6-phosphate (G6P) is an allosteric activator of glycogen synthase, promoting the glycogen synthesis process when glucose is abundant in the cell.
ATP (high energy state) also activates glycogen synthase, while AMP (low energy state) activates glycogen phosphorylase, thus inhibiting glycogenesis.
Significance of Glycogenesis
Storage of Glucose:
Glycogenesis is crucial for glucose storage in the body, particularly in the liver and muscles.
The liver stores glycogen as a reservoir to maintain blood glucose levels during periods of fasting, while muscle glycogen is used for local energy production during exercise.
Regulation of Blood Glucose:
Glycogenesis helps regulate blood glucose levels after meals. When glucose levels are high, insulin stimulates glycogenesis, which stores excess glucose in the form of glycogen.
This prevents hyperglycemia (high blood glucose levels) and ensures that glucose is available for energy when needed.
Energy Supply During Exercise:
During exercise, muscle glycogen is the primary source of glucose for ATP production. Glycogenesis ensures that muscles have enough glycogen stored for quick energy release during physical activity.
Prevention of Hyperglycemia:
Glycogenesis prevents the harmful effects of hyperglycemia by converting excess glucose into glycogen for storage, thus maintaining glucose homeostasis.
4. Glycogenolysis – Releasing Stored Glucose
Clinical Relevance:
McArdle disease: Muscle phosphorylase deficiency → exercise intolerance
Hers disease: Liver phosphorylase deficiency → hepatomegaly, hypoglycemia
📍 Location: Liver (to maintain blood glucose), muscle (for local use)
🔑 Key Enzyme: Glycogen phosphorylase🔁 Steps: Glycogen → G1P → G6P → Glucose (only in liver)
Glycogenolysis is the metabolic pathway through which glycogen (the stored form of glucose in the liver and muscles) is broken down to release glucose or glucose-6-phosphate. It is a critical process for maintaining blood glucose levels, particularly during fasting, exercise, or stress, when glucose is required rapidly by tissues
The Process of Glycogenolysis
Glycogenolysis occurs in two main steps: the breakdown of glycogen into glucose-1-phosphate and the conversion of glucose-1-phosphate to glucose (in the liver).
1. Glycogen Breakdown to Glucose-1-Phosphate
2. Remodeling of Glycogen (Debranching)
3. Conversion of Glucose-1-Phosphate to Glucose-6-Phosphate
4. Glucose Release (in the Liver)
Conclusion
Glycogenolysis is a crucial metabolic process that ensures a steady supply of glucose during periods of fasting, exercise, or stress. It is tightly regulated by hormones such as glucagon, epinephrine, and insulin, and provides a quick source of energy, particularly to the brain and muscles. Understanding the regulation and significance of glycogenolysis is essential for managing metabolic disorders, diabetes, and energy balance during exercise and fasting.
Clinical Significance of Glycogenolysis
Glycogen Storage Diseases (GSDs):
Defects in the enzymes involved in glycogenolysis, such as glycogen phosphorylase, phosphorylase kinase, or glucose-6-phosphatase, lead to glycogen storage diseases.
These diseases result in impaired glycogen breakdown, causing hypoglycemia, muscle weakness, fatigue, and liver enlargement.
Examples: GSD Type V (McArdle disease), GSD Type I (von Gierke disease).
Hyperglycemia in Diabetes:
In type 2 diabetes, insulin resistance and excessive glucagon release result in increased glycogenolysis and hepatic glucose production, contributing to hyperglycemia.
Stress and Exercise:
In conditions like stress and exercise, rapid glycogen breakdown ensures that glucose is available for immediate use by muscles and other tissues. However, excessive activation of glycogenolysis in these conditions can lead to muscle depletion or liver dysfunction over time.
Regulation of Glycogenolysis
Glycogenolysis is tightly regulated by several mechanisms to ensure glucose is released only when needed.
A. Hormonal Regulation
Glucagon (Liver)
Epinephrine (Muscles and Liver)
Insulin (Inhibition)
B. Allosteric Regulation:
AMP (Muscles)
ATP (Inhibition)
Glucose-6-Phosphate (Inhibition in Muscles)
Significance of Glycogenolysis
Maintaining Blood Glucose Levels:
Glycogenolysis is essential for maintaining blood glucose levels, especially during periods of fasting, exercise, or stress.
It provides glucose to critical tissues, particularly the brain, which depends on glucose as its primary energy source.
Energy Supply During Exercise:
During exercise, muscle glycogen stores are broken down rapidly to meet the energy demands of muscles.
In the liver, glycogen breakdown ensures a continuous supply of glucose to the bloodstream, supporting muscle activity and brain function.
Adaptation to Fasting:
In fasting conditions, glycogenolysis ensures that glucose is available to the body, especially to vital organs like the brain and red blood cells, which have limited ability to use other fuels like fatty acids.
Prevention of Hypoglycemia:
Without glycogenolysis, blood glucose levels would drop significantly during fasting or between meals, leading to hypoglycemia, which can impair brain function and lead to dizziness, confusion, and seizures.
5. HMP Shunt (Pentose Phosphate Pathway)
📍 Location: Cytoplasm (esp. in liver, RBCs, adrenal cortex)/An Alternate Route for Glucose
“More than just energy — the HMP shunt helps cells to grow, detoxify, and defend.”
🌟 Functions:
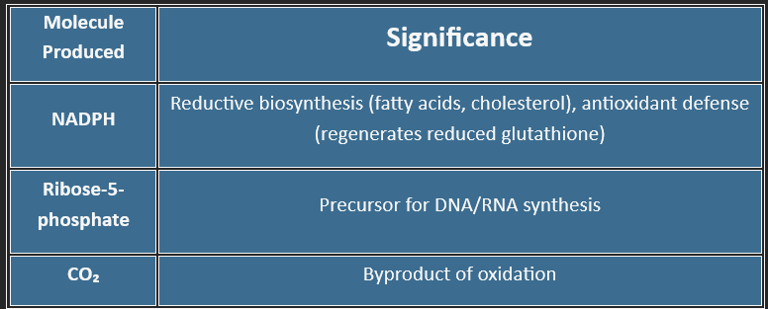
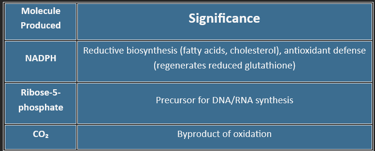
Produces NADPH (for reductive biosynthesis & GSH regeneration)
Generates ribose-5-phosphate (for nucleotide synthesis)
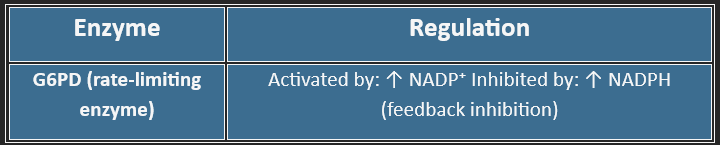
🔑 Key Enzyme: G6PD (Glucose-6-phosphate dehydrogenase)
Definition
The Hexose Monophosphate Shunt (HMP Shunt) is an alternative oxidative pathway of glucose metabolism that primarily produces:
NADPH (for biosynthesis & antioxidant defense)
Ribose-5-phosphate (for nucleotide synthesis)
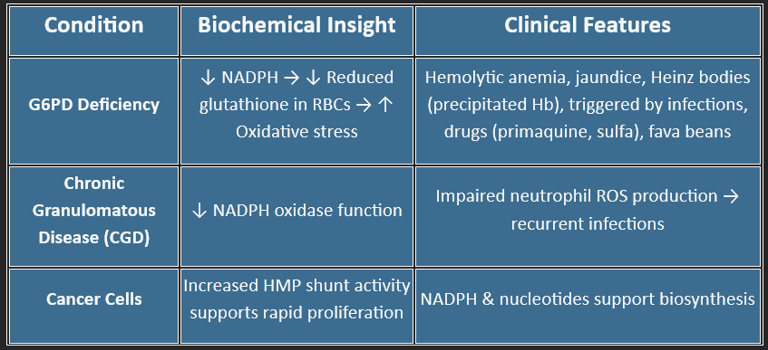
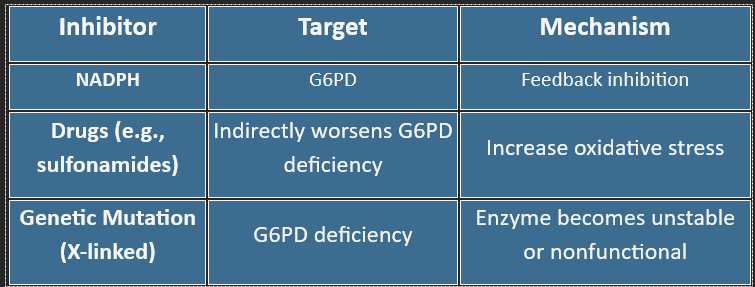
Clinical Relevance:
G6PD deficiency → hemolysis under oxidative stress (fava beans, infections, sulfa drugs)
Sites of Activity
Step-by-Step Pathway of HMP Shunt
The HMP shunt has two distinct phases:
✅ 1. Oxidative Phase (Irreversible)
Produces NADPH and Ribulose-5-phosphate
(Products: 2 NADPH + 1 Ribulose-5-phosphate + 1 CO₂)
✅2. Non-Oxidative Phase (Reversible)
It interconverts sugars to meet cellular needs.
End Products: F6P and G3P → Re-enter glycolysis or gluconeogenesis


The oxidative phase is regulated, while the non-oxidative phase is flexible and reversible to meet metabolic demands.
Significance of HMP Shunt
Energetics of HMP Shunt
The HMP shunt does not directly produce or consume ATP.
However, it produces 2 NADPH per glucose-6-phosphate oxidized.
| Glucose-6-P → 2 NADPH + 1 Ribose-5-P + 1 CO₂ |
Biomedical Importance
❌ Inhibitors of the HMP Shunt
Functions of HMP Shunt
💥 Provides NADPH for:
Fatty acid synthesis (liver, adipose)
Steroid synthesis (adrenal, testes, ovaries)
Detoxification (cytochrome P450)
Maintaining reduced glutathione (GSH) in RBCs
🧬 Produces Ribose-5-phosphate for:
DNA/RNA synthesis
Rapid cell division (marrow, tumors)
🛡️ Helps defend against oxidative stress, especially in RBCs
🧪 Supports anabolism, not catabolism

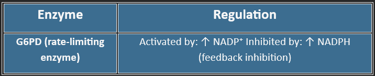
Regulation of the HMP Shunt
🌿 6. Uronic Acid Pathway (Glucuronic Acid Pathway)
📍 Location: Primarily in the liver/Cellular location: Cytosol
Also occurs in the kidneys and the intestinal mucosa
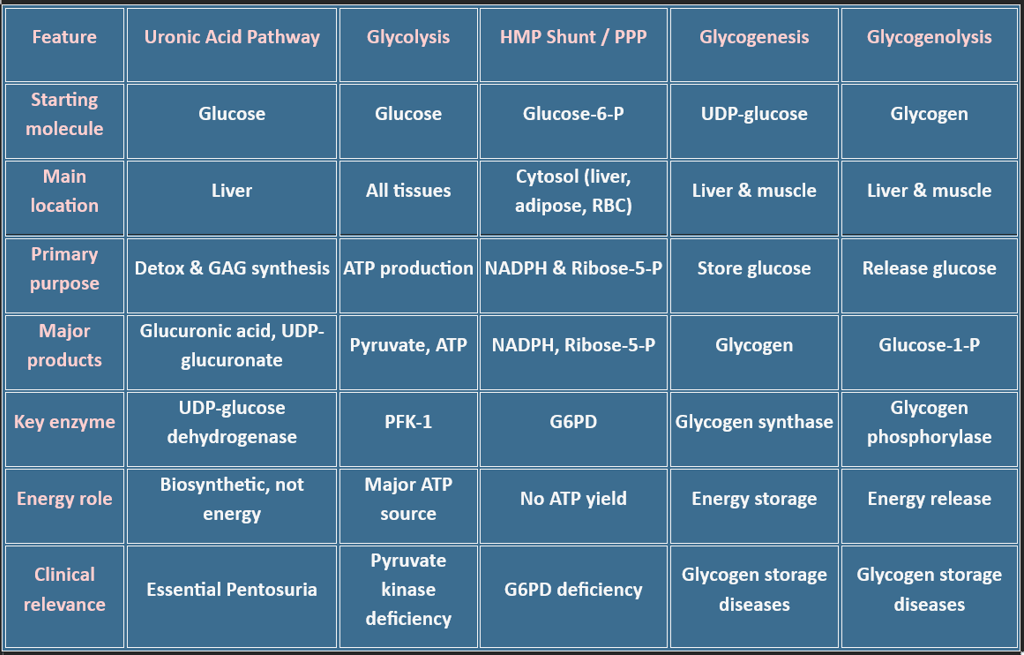
🔁 Type of Pathway
✅ Anabolic Pathway
It does not generate ATP directly.
It uses glucose derivatives to produce glucuronic acid and other biosynthetic precursors.
Mainly supports biosynthesis, detoxification, and conjugation.
Purpose of the Pathway
Converts glucose to glucuronic acid, which is used for:
Conjugation of bilirubin, drugs, and steroids
Formation of glycosaminoglycans (hyaluronic acid, heparan sulfate)
Produces ascorbic acid in most animals (not in humans)
Why is it part of Carbohydrate Metabolism?
Starts from glucose, just like glycolysis and the pentose phosphate pathway.
Converts glucose into glucuronic acid and produces:
UDP-glucuronic acid
UDP-xylose
L-xylulose
These products are essential for:
Detoxification (via glucuronidation)
Synthesis of glycosaminoglycans (e.g., hyaluronic acid, chondroitin sulfate)
Minor role in fructose formation (not significant in humans)
Clinical Relevance
Impaired bilirubin conjugation → Jaundice
Important in neonatal physiology
Defects can lead to essential Pentosuria (L-xylulose accumulation)
🌿 7. Pyruvate Metabolism
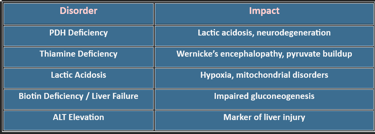
🩺 Clinical Relevance: Pyruvate dehydrogenase deficiency → lactic acidosis, neurological defects
Cori cycle: Lactate from muscles → liver → glucose
1. What is Pyruvate Metabolism?
Pyruvate is the end product of glycolysis and acts as a metabolic crossroads. Its fate depends on oxygen availability and cellular needs. Understanding pyruvate metabolism is crucial because it links glycolysis, the TCA cycle, gluconeogenesis, and amino acid metabolism.
2. Pyruvate Oxidation – Why Catabolic?
Reaction: Oxidative decarboxylation of pyruvate (3C) → Acetyl-CoA (2C) + CO₂ + NADH
Enzyme: Pyruvate Dehydrogenase Complex (PDH)
Cofactors: Thiamine (B1), NAD⁺, FAD, CoA, Lipoic acidReason it’s catabolic:
Breaks down pyruvate for energy
Generates NADH → enters ETC for ATP
Irreversible step committing carbon to energy production
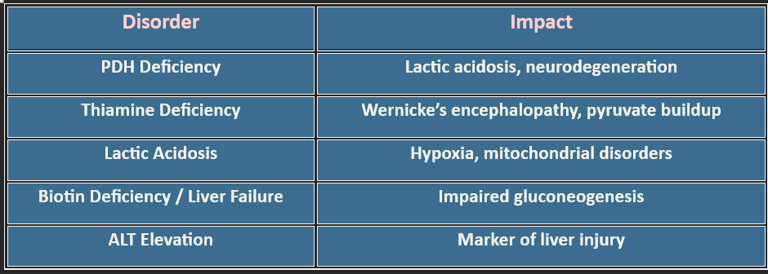
✅ Clinical Note: PDH or thiamine deficiency → pyruvate shunted to lactate → lactic acidosis.
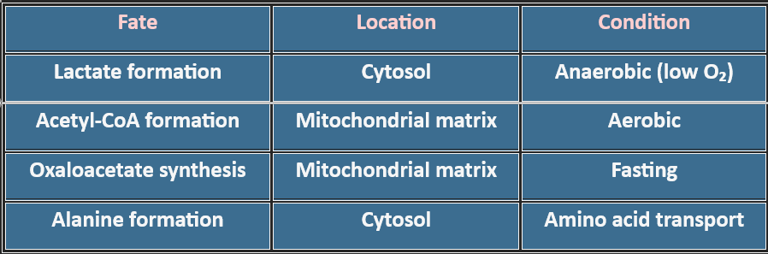
3. Where Does Pyruvate Go?
6. Clinical Correlations

4. Amphibolic Nature
Pyruvate metabolism is amphibolic:
Catabolic: Energy generation via Acetyl-CoA → TCA cycle
Anabolic: Precursor for oxaloacetate (gluconeogenesis), alanine, fatty acids
📌 Key Concept: It bridges energy-yielding and biosynthetic pathways.
5. Biological Importance
Central Hub: Connects glycolysis, TCA, gluconeogenesis, and amino acid synthesis
Energy Production: PDH reaction is irreversible → commits carbon to ATP generation
Redox Balance: Lactate formation regenerates NAD⁺ for glycolysis
Gluconeogenesis: Oxaloacetate formation during fasting maintains blood glucose
7. Therapeutic Insights
PDH Deficiency: Ketogenic diet, thiamine supplementation
Lactic Acidosis: Oxygen therapy, bicarbonate correction
Mitochondrial Disorders: Coenzyme Q10, riboflavin support
Glucose Dysregulation: Dietary and insulin management
Key Takeaway
Pyruvate metabolism is not just biochemistry—it’s clinical medicine. It explains why thiamine deficiency causes neurological symptoms, why lactate rises in hypoxia, and how metabolic therapies work.
🌿8. TCA Cycle (Citric Acid Cycle / Krebs Cycle)
📍 Location: Mitochondrial Matrix (in all aerobic cells except mature RBCs, which lack mitochondria)
2. What the TCA Cycle Actually Does
Think of the TCA cycle as a metabolic roundabout. Everything—carbs, fats, and amino acids—feeds into it as Acetyl-CoA. The cycle moves carbon around, releasing CO₂ and capturing high-energy electrons in NADH and FADH₂.
Why the cycle is considered amphibolic
It has two roles happening simultaneously:
✔ Breaks molecules down (catabolism)
• Acetyl-CoA is oxidized
• NADH, FADH₂, and GTP are formed
✔ Builds important molecules (anabolism)
TCA intermediates are used to form:
• Certain amino acids
• Glucose (via OAA)
• Heme (from succinyl-CoA)
Understanding how cells extract energy from nutrients is essential in biochemistry and clinical medicine. The TCA cycle and oxidative phosphorylation work together to convert metabolic fuel into ATP—the energy currency your cells depend on.
Nature of the Pathway: ✅ Amphibolic
Catabolic: Oxidizes Acetyl-CoA to CO₂ → produces NADH, FADH₂, and GTP (used for ATP generation).
Anabolic: Intermediates serve as precursors for biosynthesis (e.g., amino acids, heme, gluconeogenesis).
1. Where These Pathways Occur
TCA Cycle
• Takes place inside the mitochondrial matrix
• Active in all tissues that rely on oxygen
• Absent in mature RBCs, which lack mitochondria
Oxidative Phosphorylation
• Occurs on the inner mitochondrial membrane
• Requires an intact membrane and oxygen
• Uses NADH and FADH₂ generated from the TCA cycle
3. Key Reactions in the Cycle
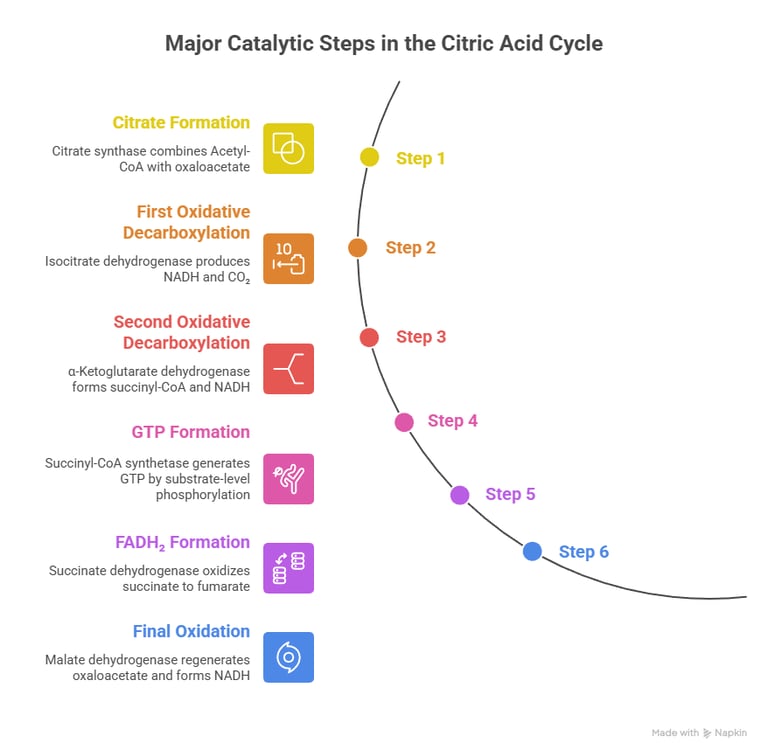
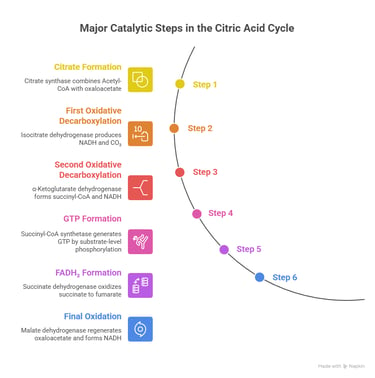
Here’s a simple walk-through of the major catalytic steps that matter most clinically:
1️⃣ Formation of Citrate
Citrate synthase combines Acetyl-CoA with oxaloacetate
→ Entry point for carbon into the cycle
2️⃣ First oxidative step
Isocitrate dehydrogenase produces the first NADH and removes CO₂
→ This is the rate-limiting step
3️⃣ Second oxidative decarboxylation
α-Ketoglutarate dehydrogenase forms succinyl-CoA and another NADH
→ Enzyme resembles PDH and uses the same cofactors
4️⃣ GTP formation
Succinyl-CoA synthetase generates GTP by substrate-level phosphorylation
5️⃣ Formation of FADH₂
Succinate dehydrogenase oxidizes succinate to fumarate
→ This enzyme is unique because it is also Complex II of the ETC
6️⃣ Final oxidation
Malate dehydrogenase regenerates oxaloacetate and forms the last NADH
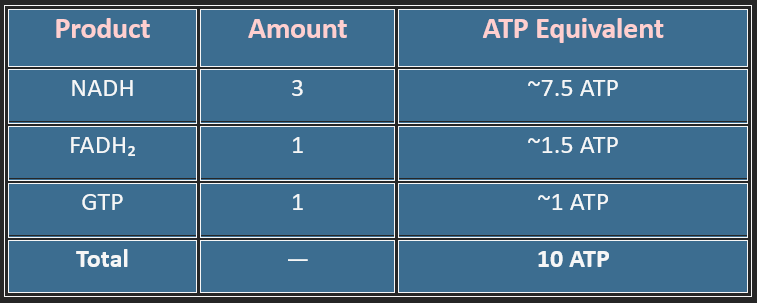
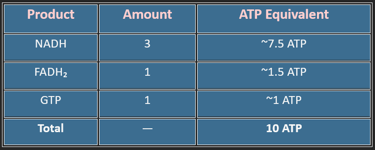
4. Energy Yield (Per Acetyl-CoA)
A single glucose gives two acetyl-CoA → ~20 ATP from the TCA cycle alone.
5. Why the Cycle Matters Biologically
A metabolic intersection
It is the final pathway for the complete oxidation of:
• Glucose
• Fatty acids
• Ketone bodies
• Certain amino acids
Source of key building blocks
Intermediates leave the cycle to form:
• Glucose (OAA → PEP → gluconeogenesis)
• Neurotransmitters (α-ketoglutarate → glutamate)
• Porphyrins/heme (succinyl-CoA)
Supports aerobic energy production
Nearly all NADH and FADH₂ used by the ETC originate here.
6. Regulation — What Turns the Cycle Up or Down
Stimulated by
• ADP (signals low energy)
• Calcium (important in active muscle)
• Increased acetyl-CoA availability
Inhibited by
• High ATP
• Excess NADH
• Succinyl-CoA buildup
• Citrate accumulation
8. Clinical Connections
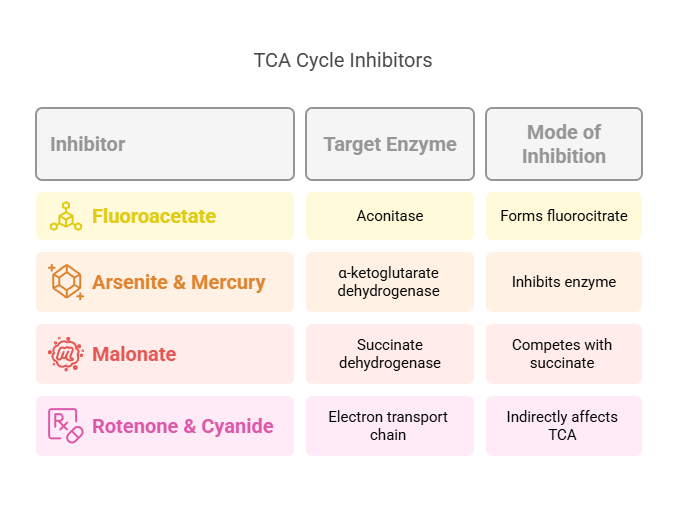
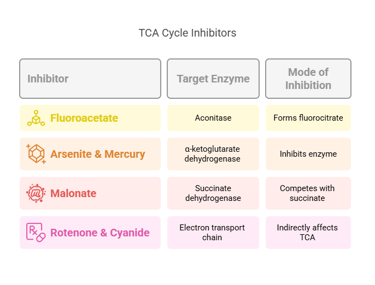
1. Thiamine deficiency (Vitamin B1)
• PDH and α-ketoglutarate dehydrogenase become impaired
• Pyruvate and lactate accumulate
• Seen in Wernicke–Korsakoff syndrome and chronic alcoholism
2. Arsenic toxicity
• Blocks lipoic acid
• Disrupts PDH and α-KG dehydrogenase
• Severe inhibition of aerobic metabolism
3. Inherited mitochondrial enzyme defects
• Mutations in succinate dehydrogenase or other complex proteins
• Present with muscle weakness, neurological symptoms, and lactic acidosis
4. Mitochondrial myopathies
• Reduced oxidative ATP production
• Exercise intolerance, early fatigue
7. Oxidative Phosphorylation — Where ATP Is Actually Made
The reduced coenzymes (NADH, FADH₂) feed electrons into the electron transport chain (ETC) embedded in the inner mitochondrial membrane.
How it works
Electrons move through Complex I → IV.
Protons are pumped into the intermembrane space.
A proton gradient is created.
ATP synthase (Complex V) uses this gradient to make ATP.
Oxygen is the final electron acceptor
• Without oxygen, the chain stops
• NADH accumulates
• The TCA cycle slows
• Anaerobic glycolysis takes over
9. Quick Student Summary
1. The TCA cycle runs in the mitochondrial matrix and generates high-energy electron carriers.
2.It is amphibolic, fueling both energy release and molecule synthesis.
3. Oxidative phosphorylation uses NADH/FADH₂ to create most of the body’s ATP.
4. Many clinical disorders arise from defects in dehydrogenases or mitochondrial function.
🌿 9. Oxidative Phosphorylation (Electron Transport Chain)
📍 Location: Inner Mitochondrial Membrane/Pathway: ✅ Strictly Catabolic
Produces >90% of cellular ATP in aerobic cells. /Oxygen is the final electron acceptor → forms water.
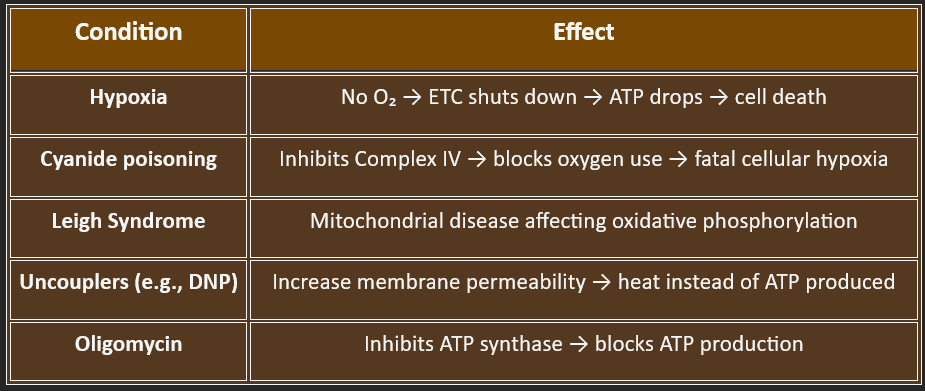

Process Summary
Electrons from NADH & FADH₂ pass through Complexes I–IV, releasing energy.
This energy pumps H⁺ ions into the intermembrane space, creating a proton gradient.
ATP synthase (Complex V) uses this gradient to produce ATP from ADP + Pi.
Biological Significance
Converts chemical energy from nutrients into ATP, the universal energy currency.
Essential for cell survival, especially in high-energy organs (brain, heart, muscle).
Also generates heat in brown adipose tissue (non-shivering thermogenesis via uncoupling proteins).
⚕️ Clinical Significance
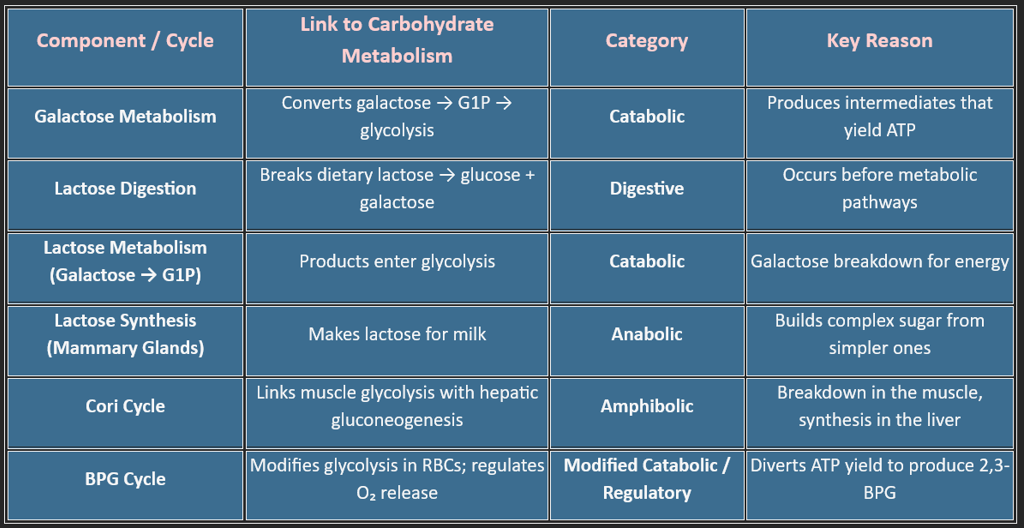
10. Other metabolic pathways include: (Fructose, Galactose, Lactose, Cori cycle, BPG Cycle)
Carbohydrate metabolism is more than just glycolysis and the TCA cycle — it includes several specialized pathways that help the body adapt to dietary variations, oxygen availability, tissue needs, and red-blood-cell physiology.
Among these, Galactose metabolism, Lactose digestion, the Cori cycle, and the BPG cycle play unique roles in maintaining energy balance, glucose regulation, and metabolic flexibility.
1. Galactose Metabolism (The Leloir Pathway)
Galactose is obtained mainly from lactose, making its metabolism essential for infants, children, and adults with dairy intake.
Why It Belongs to Carbohydrate Metabolism
Galactose is converted into glucose-1-phosphate (G1P) and then into glucose-6-phosphate (G6P), a central glycolytic intermediate.
Thus, it feeds directly into glycolysis, glycogenesis, or the pentose phosphate pathway.
Key Enzymes :
Galactokinase (GALK) – phosphorylates galactose
GALT (Galactose-1-phosphate uridyltransferase) – core step
GALE (UDP-galactose 4’-epimerase) – regenerates UDP-glucose
2. Lactose — Digestion, Absorption & Metabolic Fate
Lactose itself is not a metabolic pathway but a substrate that connects digestion with carbohydrate metabolism.
2A. Lactose Digestion (Small Intestine)
Key Enzyme
✔ Lactase (β-galactosidase) on the intestinal brush border.
Products
Lactose → Glucose + Galactose
Classification/Type✔ Digestive process, not metabolic
(because it happens before absorption and before cellular metabolism begins)
2B. Fate of Glucose and Galactose After Absorption
Once absorbed:
Glucose
→ enters glycolysis, glycogenesis, or HMP shunt
Galactose
→ goes through the Leloir pathway, ultimately forming G1P → G6P
Classification/Type
✔ Catabolic, because absorbed monosaccharides are broken down for energy.
1. Galactose Metabolism
2. Lactose — Digestion, Absorption & Metabolic Fate
Location:
✔ Liver (primary)
✔ Intestinal mucosa
✔ Ovaries and other tissues (minor)
Overall Process :
Galactose → Galactose-1-phosphate → UDP-galactose → UDP-glucose → G1P → Glycolysis
Classification/Type:
✔ Catabolic
Because the pathway breaks down galactose to produce intermediates used for ATP generation.
Clinical Note: Galactosemia
A defect in GALT leads to accumulation of toxic metabolites → jaundice, liver failure, cataracts.
2C. Lactose Synthesis (Mammary Gland): Occurs only during lactation.
Substrates: Glucose + UDP-Galactose
Key Enzyme: Lactose synthase (α-lactalbumin + galactosyltransferase)
Classification/Type ✔ Anabolic, because it builds a complex molecule from simple sugars.
3. The Cori Cycle — Muscle ↔ Liver Energy Shuttle
The Cori cycle explains how the body recycles lactate produced during anaerobic glycolysis, especially:
1. Strenuous exercise, 2. Hypoxia, 3. RBC metabolism (since RBCs lack mitochondria)
How the Cycle Works?
In Muscle (Anaerobic Glycolysis)
Glucose → Pyruvate → Lactate
Lactate leaves the muscle and enters the blood.
In Liver
Lactate → Pyruvate → Glucose (via gluconeogenesis)
The newly formed glucose returns to the muscle → reused for ATP generation.
Why is it part of Carbohydrate Metabolism?
Because it connects glycolysis (in muscle) to gluconeogenesis (in liver), allowing continuous ATP production even without oxygen.
Classification/Type ✔ Amphibolic
• Catabolic in muscle (breakdown of glucose)
• Anabolic in the liver (glucose synthesis)
Physiological Importance: 1. Prevents lactic acidosis, 2. Maintains blood glucose during exercise, 3. Supports RBC metabolism, since RBCs always produce lactate
This special pathway exists only in red blood cells, which lack mitochondria and depend entirely on glycolysis.
1. What the BPG Cycle Does: It diverts 1,3-BPG, a glycolytic intermediate, to form 2,3-BPG (2,3-DPG).
2. Why 2,3-BPG Is Crucial? It binds hemoglobin and reduces oxygen affinity, allowing oxygen release to tissues —
especially important during:
✔ High altitude
✔ Anemia
✔ Exercise
✔ Chronic hypoxia
3. Metabolic Consequence: Because the cycle bypasses ATP-generating steps, RBCs sacrifice energy to maintain proper oxygen delivery.
4. Classification/Type: ✔ Modified Catabolic / Regulatory Side-Branch
Not fully catabolic because:
a) It does not mainly exist to generate ATP, b) It modulates hemoglobin function, c) It slightly alters glycolytic ATP yield
4. BPG Cycle (Rapoport–Luebering Shunt) — RBC Oxygen Regulation Pathway
5. Expanded Comparative Classification Table
6. Final Summary
1. Lactose digestion is a digestive process, not metabolic.
2. Galactose metabolism is catabolic because it feeds into glycolysis.
3. Lactose synthesis during lactation is anabolic.
4. The Cori cycle acts as a recycling loop between the muscle and liver (amphibolic).
5. The BPG cycle is a specialized side-path in RBCs regulating oxygen delivery.
BLOG
Join us to explore medical biochemistry intricacies.
WRITE TO US
© 2024. All rights reserved.
