Bioenergetics & components
Definition:
Bioenergetics is the study of how living organisms acquire, convert, store, and utilize energy to perform biological work, such as muscle contraction, active transport, biosynthesis, and maintenance of cellular homeostasis.
In simpler terms: -
Bioenergetics explains how your body obtains energy from nutrients (such as glucose, fats, and proteins), and how this energy is utilized to power various bodily functions, including cell division, the heartbeat, and brain function.
'Why It Matters in Medicine'
🎯Helps understand how ATP (adenosine triphosphate) is made and used.
🎯Forms the foundation of metabolism, nutrition, and physiology.
🎯Crucial for understanding disorders involving mitochondrial function, metabolic syndromes, and energy deficits in diseases.
It is the branch of biochemistry and cell biology that deals with the study of energy flow and transformation in biological systems.
⚙️ Main Components of Bioenergetics:
The main components of bioenergetics, including key systems and concepts, are as follows: -
1. 🔋 Energy Currency – ATP (Adenosine Triphosphate)
Central molecule for energy transfer in cells
Stores energy in high-energy phosphate bonds
Hydrolysis of ATP → ADP + Pi releases energy for cellular work.
Regenerated through catabolic pathways (e.g., glycolysis, TCA, ETC)
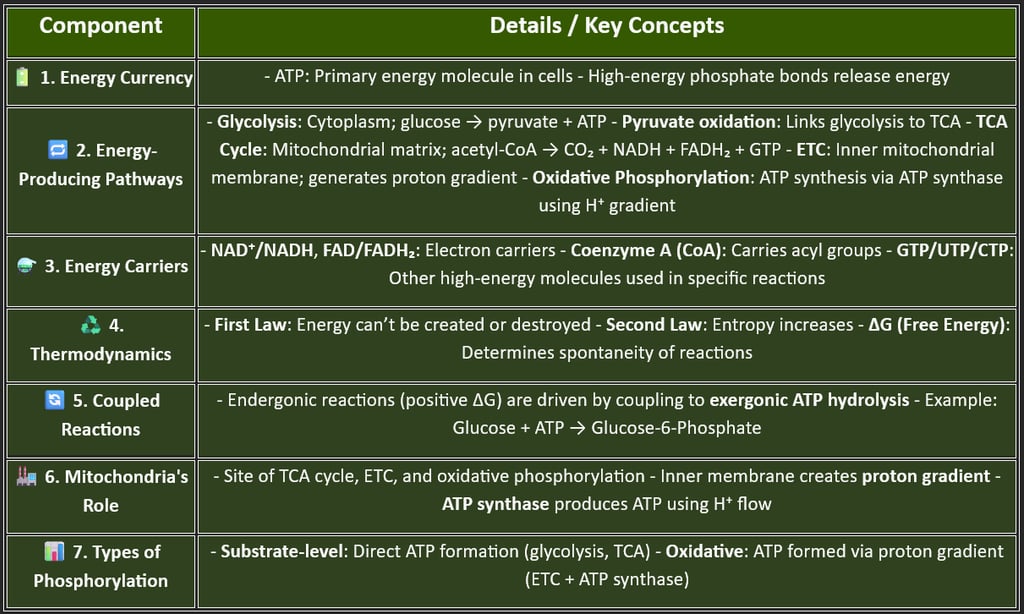
3. 🔋Energy-Carrying Molecules
These molecules transfer electrons or energy between reactions:
NAD⁺/NADH
FAD/FADH₂
Coenzyme A (CoA)
GTP/UTP/CTP (minor energy currencies)
4. Thermodynamics in Biological Systems
Core principles that govern energy transformations:
First Law: Energy cannot be created or destroyed — only converted
Second Law: Energy transfer increases entropy
Free Energy Change (ΔG):
Negative ΔG → Spontaneous (exergonic)
Positive ΔG → Requires input (endergonic)
6. ⚡ Substrate-Level Phosphorylation vs. Oxidative Phosphorylation
2. Energy-Producing Pathways (Catabolism)
These metabolic pathways break down nutrients to release energy, mainly in the form of ATP:
a. Glycolysis
Occurs in the cytoplasm
Anaerobic or aerobic
Glucose → Pyruvate + ATP + NADH
b. Pyruvate Oxidation
Converts pyruvate → Acetyl-CoA
Links glycolysis to the TCA cycle
Generates NADH
5. Coupled Reactions
Endergonic reactions (require energy) are coupled to ATP hydrolysis to become favorable
Example: Glucose phosphorylation (glucose + ATP → glucose-6-P + ADP)
7. Mitochondria – The Powerhouse
Site of the TCA cycle, ETC, and oxidative phosphorylation
The inner membrane is crucial for the proton gradient and ATP synthesis.
Contains its DNA (mtDNA), linked to many inherited metabolic disorders
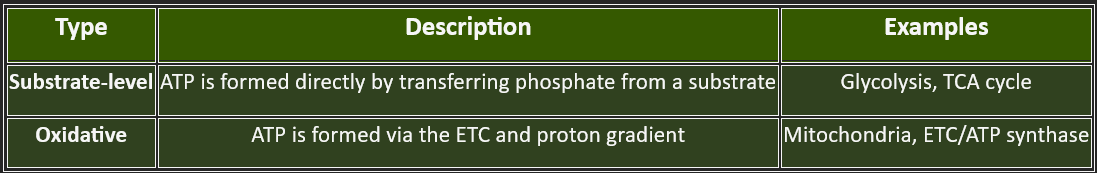
c. TCA Cycle (Krebs Cycle)
Occurs in mitochondria
Acetyl-CoA → CO₂ + NADH + FADH₂ + GTP
Main source of reduced coenzymes
d. Electron Transport Chain (ETC)
Located in the inner mitochondrial membrane
NADH/FADH₂ donate electrons → O₂ (final acceptor)
Establishes proton gradient
e. Oxidative Phosphorylation
Driven by the proton gradient
ATP synthase uses H⁺ flow to make ATP
Produces the bulk of ATP in aerobic respiration
The ETC is the powerhouse of cellular respiration. It efficiently converts the energy stored in NADH and FADH₂ into ATP to support cellular processes.
⚡ final stage of cellular respiration
⚡Location: the inner mitochondrial membrane.
⚡Function: pivotal role in oxidative phosphorylation, which generates the bulk of ATP in cells.
⚡The Electron Transport Chain (ETC)⚡
Stepwise Flow of Electrons in the ETC
Flow from High Energy to Low Energy in the Electron Transport Chain (ETC)
1. Electron Donors (High Energy):
NADH and FADH₂, generated during glycolysis, the Krebs cycle, and beta-oxidation, donate their high-energy electrons to the ETC.
2. Complex I (NADH-CoQ Reductase):
NADH donates electrons, reducing FMN and iron-sulfur (Fe-S) clusters.
High-energy electrons are transferred to Coenzyme Q (ubiquinone).
Protons (H⁺) are pumped into the intermembrane space, building the gradient.
3. Complex II (Succinate-CoQ Reductase):
Electrons from FADH₂ bypass Complex I and are transferred directly to Coenzyme Q.
Unlike Complex I, Complex II does not pump protons.
In the Electron Transport Chain (ETC), the flow of electrons follows a stepwise movement from high-energy electron carriers (NADH, FADH₂) to molecular oxygen (O₂), which has the lowest energy in the system.
This gradual transfer is essential to maximize energy efficiency and avoid harm to the cell.
Let’s explore the flow and the rationale behind this design.
What Does "High Energy to Low Energy" Mean?
Energy Levels:
Electrons in NADH and FADH₂ have high potential energy due to their reduced state.
As electrons pass through the complexes, they lose energy at each step, which is used to pump protons into the intermembrane space.
Controlled Energy Release:
Instead of releasing all the energy at once (which would cause damage), the ETC dissipates energy gradually.
Think of it like water flowing down a series of dams, each step harnessing some of the energy.
4. Coenzyme Q:
CoQ acts as a mobile carrier, shuttling electrons from Complex I and II to Complex III.
5. Complex III (Cytochrome bc1 Complex):
Transfers electrons to Cytochrome c.
Protons are pumped into the intermembrane space.
6. Cytochrome c:
A small protein that transfers electrons to Complex IV.
7. Complex IV (Cytochrome c Oxidase):
Electrons reduce oxygen (O₂), the final electron acceptor, to form water (H₂O).
Protons are pumped into the intermembrane space, further building the gradient.
🔋Significance of Controlled Energy Flow in the ETC
1. Uncontrolled Energy Release:
If the energy from NADH and FADH₂ were released all at once, it would generate excessive heat and disrupt cellular structures. This uncontrolled release would be biologically inefficient and potentially damaging.
2. Cellular Damage:
High-energy electrons can generate Reactive Oxygen Species (ROS), such as superoxide (O₂⁻•), which are harmful to DNA, proteins, and lipids. The ETC minimizes ROS production by carefully channeling electrons through the electron transport chain.
3. Thermodynamic Constraints:
Biological systems need controlled steps to maintain energy conversion efficiency. A sudden release of energy would violate these constraints and prevent ATP synthesis.
4. ATP Synthase Function:
The proton gradient created by the gradual energy transfer is critical for ATP production. Without the stepwise movement, the proton gradient (and ATP synthase) would fail, depriving the body of energy.
⚡Why Our Body Cannot Handle High Energy Directly⚡
Efficient ATP Production:
The stepwise flow ensures a sustainable and controlled energy release, driving ATP synthesis through chemiosmosis.
Reduction of Heat Loss:
By avoiding an abrupt release of energy, the ETC conserves energy for ATP production rather than losing it as heat.
ROS Prevention:
Proper electron transfer reduces the risk of oxidative stress and cellular damage.
The ETC is like an energy staircase designed to extract energy from electrons safely and efficiently. This process is finely tuned to meet cellular demands while avoiding harm.
The Electron Transport Chain (ETC)
The Electron Transport Chain (ETC) is a series of protein complexes and electron carriers located in the inner mitochondrial membrane.
It is the final stage of cellular respiration, where electrons from NADH and FADH2 are transferred to oxygen, producing water and generating ATP.
It plays a pivotal role in oxidative phosphorylation, which generates the bulk of ATP in cells.
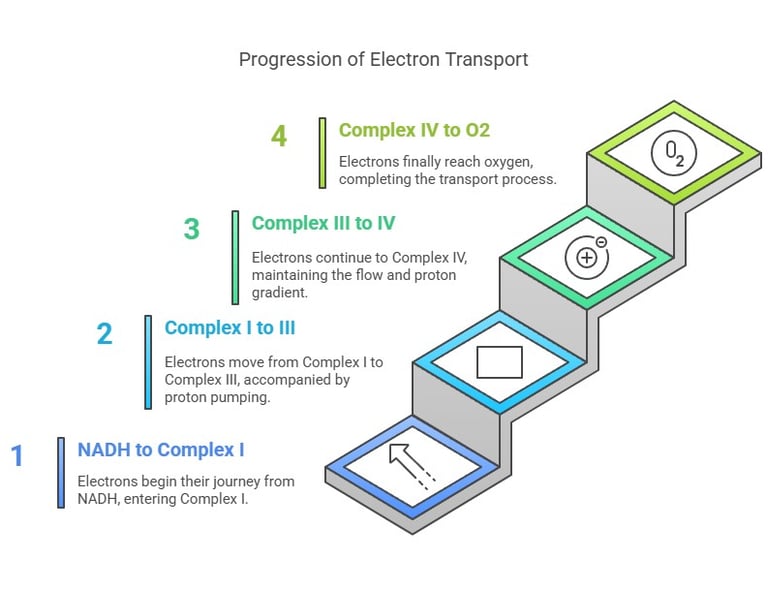
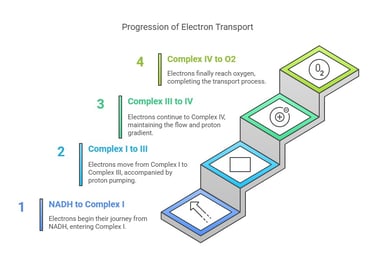
Key Components of the ETC
The ETC consists of a series of protein complexes and electron carriers.
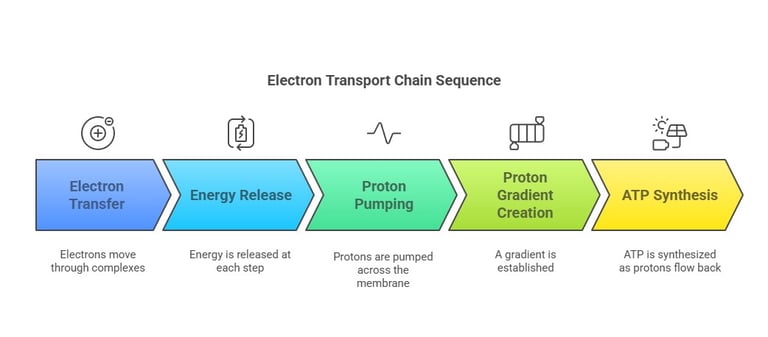
Electron Flow & Proton Pumping
Electron Movement:
Electrons travel stepwise from NADH/FADH₂ → Complex I/II → Complex III → Complex IV → O₂
Each carrier in the chain has a progressively higher redox potential, ensuring a “downhill” flow.
Proton Pumping:
As electrons flow, certain complexes (I, III, IV) pump H⁺ ions from the mitochondrial matrix into the intermembrane space.
Electron Carriers & Proteins:
NADH & Flavoproteins: Carry high-energy electrons
Non-heme Metalloproteins (Fe-S Proteins): Involved in electron transfer
Ubiquinone (Coenzyme Q): A lipid-soluble carrier anchoring to the membrane
Cytochromes: Proteins containing heme groups that shuttle electrons
The Four Main Protein Complexes
Complex I – NADH-Coenzyme Q Reductase
Complex II – Succinate Dehydrogenase
Complex III – Cytochrome c Reductase
Complex IV – Cytochrome c Oxidase
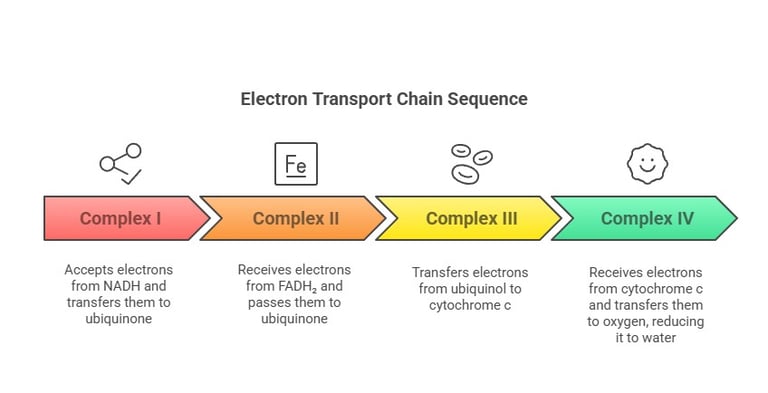
Complex I – NADH-Coenzyme Q Reductase:
Accepts electrons from NADH
Passes electrons through FMN and Fe-S clusters to ubiquinone
Complex II – Succinate Dehydrogenase:
Receives electrons from FADH₂ (from the Krebs cycle or β-oxidation)
Transfers electrons to ubiquinone
Complex III – Cytochrome c Reductase:
Transfers electrons from reduced ubiquinone (ubiquinol) to cytochrome c
Complex IV – Cytochrome c Oxidase:
Receives electrons from cytochrome c and transfers them to oxygen (O₂), reducing it to water (H₂O)
Location:
Embedded along the inner mitochondrial membrane (on the cristae)
Key Role:
Transfers electrons from energy-rich carriers (NADH and FADH₂) to oxygen (O₂)
Uses the energy from these electrons to pump H⁺ ions across the membrane
Result/the outcome:
A proton gradient (electrochemical potential) is established to drive ATP synthesis
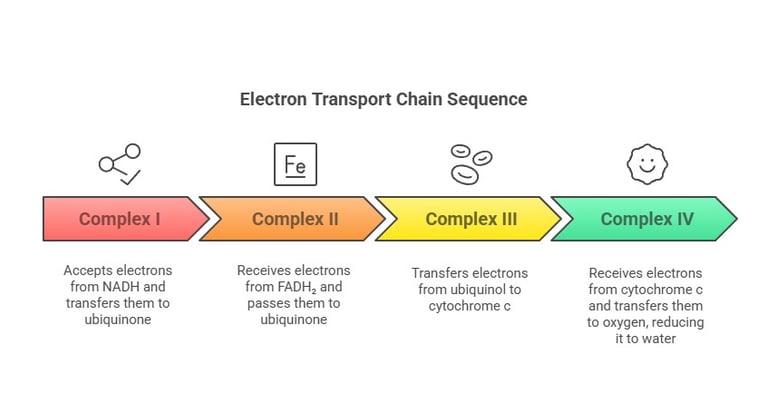
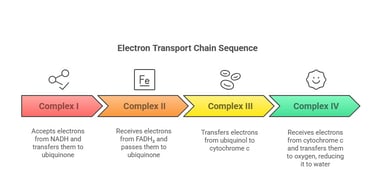
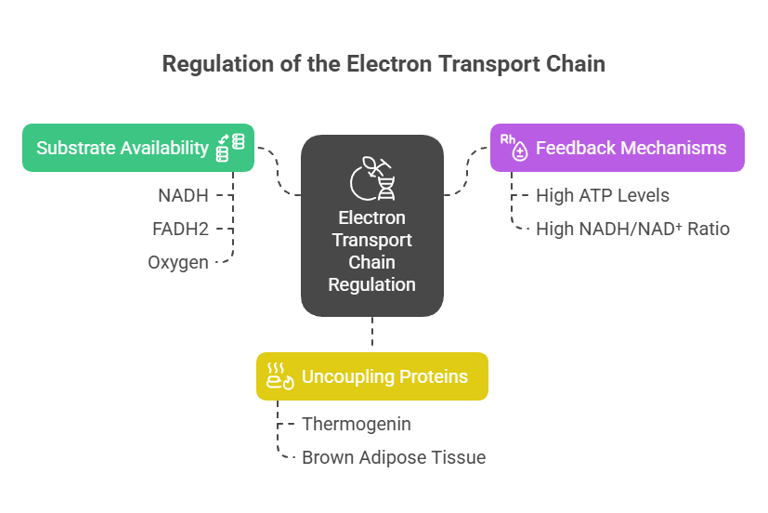
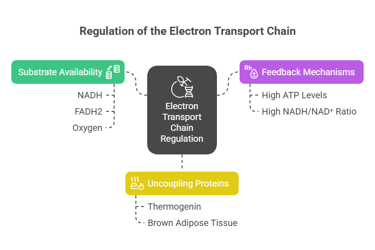
Regulation and Inhibition of the ETC
1. Substrate Availability:
Requires NADH, FADH2, and oxygen for electron transfer.
Limited oxygen (e.g., during hypoxia) slows the chain.
2. Feedback Regulation:
ATP levels regulate the activity; high ATP concentrations inhibit the ETC, while high ADP levels stimulate it.
3. Role of Uncouplers:
Uncoupling proteins (e.g., thermogenin) allow protons to return to the matrix without generating ATP, dissipating energy as heat (important in brown adipose tissue).
Inhibition of the ETC
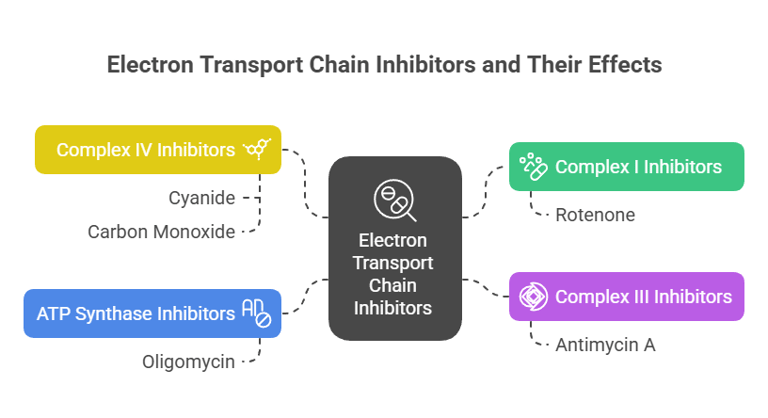
1. Complex Inhibitors:
Rotenone: Inhibits complex I.
Antimycin A: Inhibits complex III.
Cyanide or Carbon Monoxide: Inhibits complex IV, blocking oxygen reduction and halting the chain.
2. Disruptions:
Lack of oxygen (e.g., ischemia) halts ETC function, leading to reliance on anaerobic metabolism.
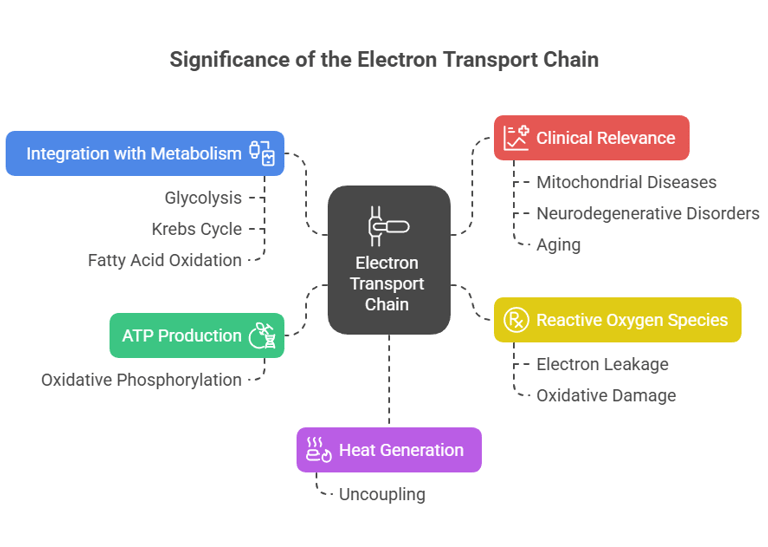
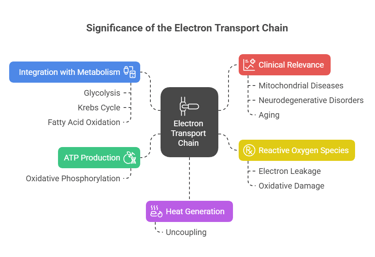
1. ATP Production:
The ETC generates the majority of ATP in aerobic organisms through oxidative phosphorylation.
2. Heat Generation:
Uncoupling of the ETC produces heat, aiding in thermoregulation.
3. Reactive Oxygen Species (ROS):
Electron leakage can produce ROS, which are involved in cell signaling but can also cause oxidative damage if uncontrolled.
4. Integration with Metabolism:
Links glycolysis, the Krebs cycle, and fatty acid oxidation.
5. Clinical Relevance:
Dysfunctions in the ETC are associated with mitochondrial diseases, neurodegenerative disorders, and aging.
The ETC is tightly controlled to meet cellular energy demands:
Significance of the ETC
BLOG
Join us to explore medical biochemistry intricacies.
WRITE TO US
© 2024. All rights reserved.
