Acid-Base Balance
The regulation of acid-base balance involves three integrated defense systems: immediate chemical buffers, rapid respiratory compensation, and long-term renal regulation. The bicarbonate buffer system, governed by the Henderson-Hasselbalch equation, is the primary extracellular buffer.
Acid–Base Regulation – Definition
Acid–base regulation is the physiological process by which the body maintains the hydrogen ion concentration [H+][H^+] of body fluids within a narrow range (arterial pH 7.35–7.45), despite continuous production of acids and bases during metabolism.
This regulation involves the coordinated action of:
Chemical Buffer Systems (Immediate Response)
Respiratory system – within minutes.
Renal system – from hours to days.
Significance of Acid–Base Regulation
Maintains optimal pH for enzyme activity, cell function, electrolyte balance, and overall homeostasis. Maintaining pH isn’t just academic — it’s life-critical:
Normal pH range (7.35–7.45) ensures proper cell function.
Acidosis (pH < 7.35) → depresses CNS, impairs cardiac contractility, and can cause coma.
Alkalosis (pH > 7.45) → increases neuromuscular excitability, can cause seizures.
Electrolyte balance: pH changes affect K⁺, Ca²⁺, and phosphate levels.
Metabolic coordination: pH influences hormone binding, substrate utilization, and activation of metabolic pathways.
Functions of Acid–Base Regulation Systems
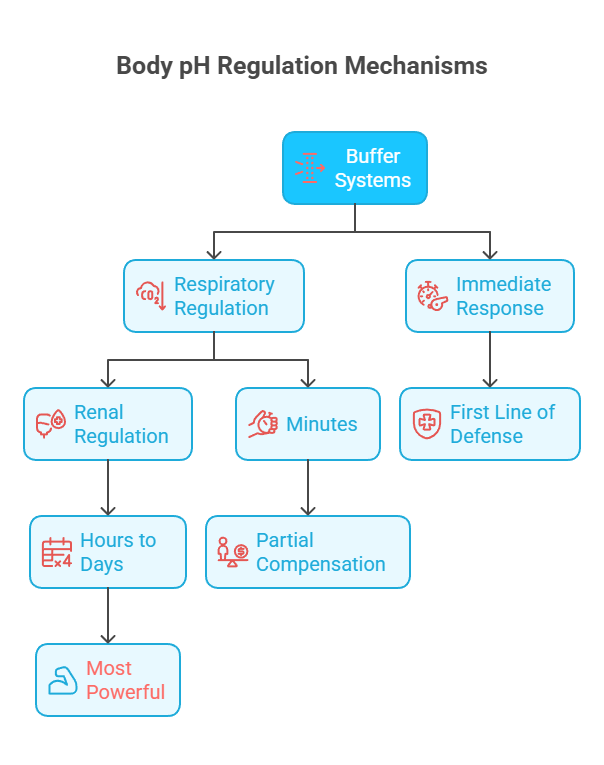
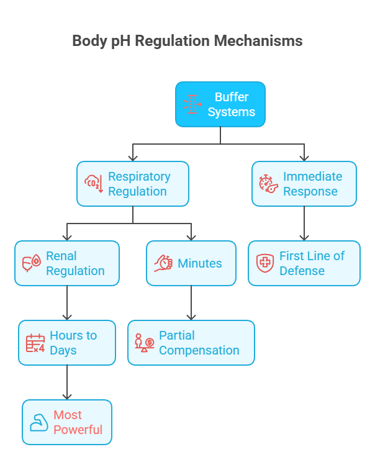
The body has a three-tier defense system:
Chemical Buffers (seconds) → Immediate pH stabilization. Minimize sudden pH changes. These are weak acid-conjugate base pairs that resist changes in pH by absorbing excess hydrogen or hydroxyl ions.
Respiratory Regulation (minutes) → Adjusts CO₂ removal via breathing rate/depth. Adjusts CO₂ excretion within minutes.
Renal Regulation (hours to days) → Excretes H⁺, regenerates and reabsorbs HCO₃⁻. Adjusts H⁺ excretion and HCO₃⁻ reabsorption/generation over hours to days.
Together, they minimize pH changes despite ongoing acid-base production.
Importance of Acid-Base Balance
The maintenance of proper acid-base balance is critical for normal physiological function. The extracellular fluid, particularly blood plasma, must maintain a pH between 7.35-7.45 for optimal cellular metabolism and enzyme function. Even minor deviations from this range can severely affect organ function, as proteins are denatured and enzymes lose their activity outside the acceptable pH range.
The Mechanism of Regulation of Acid–Base Balance
The body maintains pH by three coordinated mechanisms:
A) Buffer systems: (immediate response — seconds) (especially bicarbonate)
B) Respiratory regulation of pH: (minutes) (via CO₂ control)
C) Renal (metabolic) regulation: (hours to days) (via H⁺ and HCO₃⁻ handling)
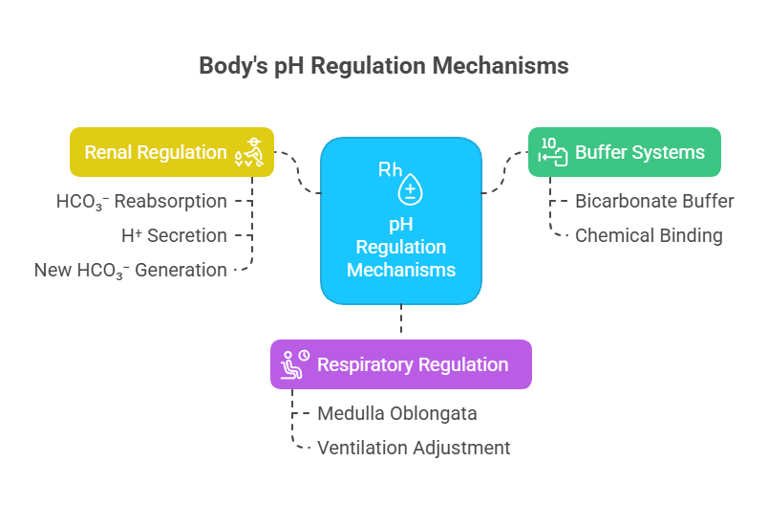
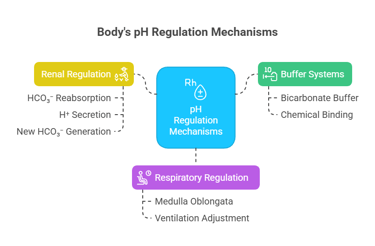
A) Buffer systems (immediate response — seconds)
First line of defense.
Chemical buffers bind or release H⁺ instantly.
Example: Bicarbonate buffer in blood.
B) Respiratory regulation of pH (minutes)
Controlled by the respiratory center in the medulla oblongata.
If pH ↓ (acidosis):
Peripheral & central chemoreceptors sense ↑[H⁺] or ↑PaCO₂.
Ventilation ↑ → more CO₂ exhaled → reaction shifts left → ↓H⁺ → pH rises.
If pH ↑ (alkalosis):
Ventilation ↓ → CO₂ retained → reaction shifts right → ↑H⁺ → pH drops.
Speed: acts within minutes; partial compensation.
C) Renal (metabolic) regulation (hours to days)
Kidneys adjust pH by:
Reabsorbing filtered HCO₃⁻ (mainly proximal tubule).
Secreting H⁺ into urine (distal tubule/collecting duct).
Generating new HCO₃⁻ via ammonium (NH₄⁺) excretion and phosphate buffering in urine.
Slowest but most powerful long-term mechanism.
Key Takeaway:
pH homeostasis is like a 3-tier defense:
1. Buffers — instant but limited.
2. Lungs — quick, medium capacity.
3. Kidneys — slow but most powerful.
Disorders of Acid–Base Balance
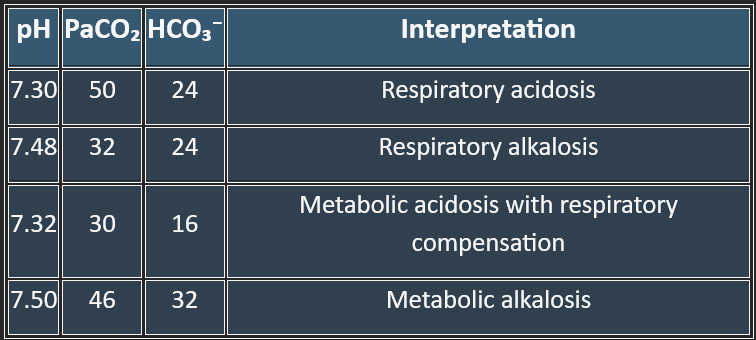
Two main types: Acidosis (pH < 7.35) and Alkalosis (pH > 7.45).
Each can be metabolic or respiratory, giving 4 primary disorders.
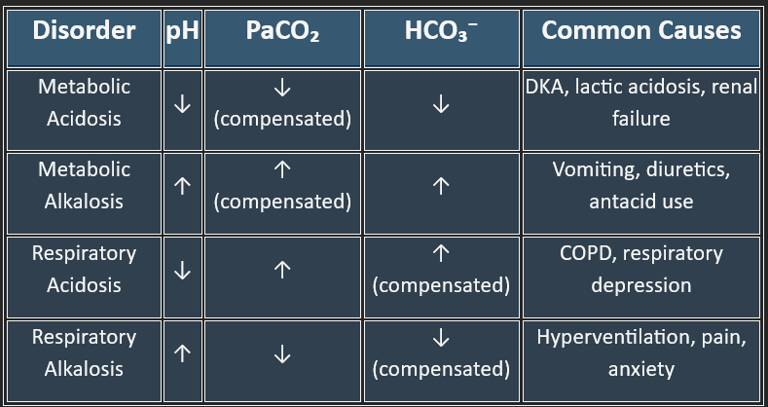
A) Metabolic Acidosis
B) Metabolic Alkalosis
C) Respiratory Acidosis
D) Respiratory Alkalosis
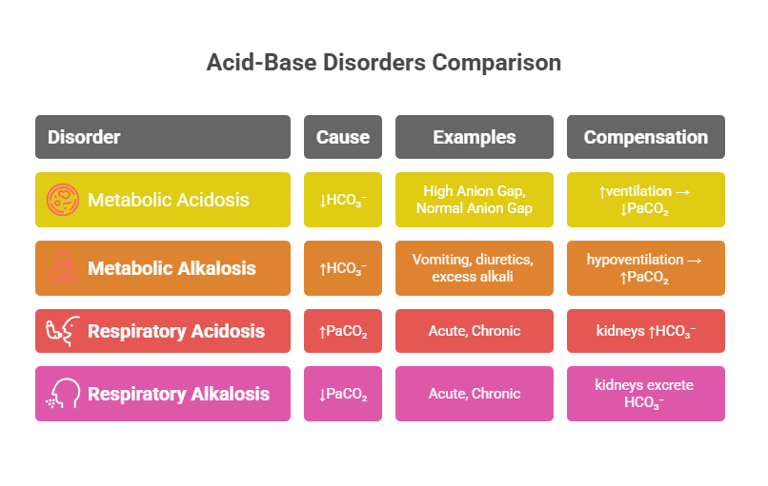
B) Metabolic Alkalosis
Cause: ↑HCO₃⁻ (primary disturbance).
Examples: vomiting (loss of gastric acid), diuretics, excess alkali ingestion.
Compensation: hypoventilation → ↑PaCO₂ (limited by hypoxia drive).
A) Metabolic Acidosis
Cause: ↓HCO₃⁻ (primary disturbance).
Examples:
High Anion Gap: lactic acidosis, diabetic ketoacidosis, renal failure, poisoning (methanol, ethylene glycol).
Normal Anion Gap: diarrhea, renal tubular acidosis.
Compensation: ↑ventilation → ↓PaCO₂.
C) Respiratory Acidosis
Cause: ↑PaCO₂ (primary disturbance).
Acute: CNS depression (drugs, trauma), airway obstruction.
Chronic: COPD, neuromuscular disorders.
Compensation: kidneys ↑HCO₃⁻ (slow).
D) Respiratory Alkalosis
Cause: ↓PaCO₂ (primary disturbance).
Acute: hyperventilation from anxiety, pain, fever, hypoxia.
Chronic: high altitude.
Compensation: kidneys excrete HCO₃⁻.
Understanding acid-base balance is crucial because it reflects the body's ability to maintain a stable pH, a key determinant of enzyme function, cellular stability, and overall homeostasis. The body uses three main systems to regulate pH.
When these systems are disturbed, we observe acidosis (pH < 7.35) or alkalosis (pH > 7.45), resulting from either respiratory or metabolic disturbances. Arterial blood gas (ABG) analysis helps us figure out what’s going wrong.
Clinical Significance in Dentistry
For dental students, understanding acid-base balance is particularly relevant because:
Salivary pH affects oral health and caries development
Demineralization of tooth enamel occurs at a pH ≤ 5.5
Periodontal disease is associated with altered salivary pH
Systemic diseases affecting acid-base balance may manifest in oral symptoms
BLOG
Join us to explore medical biochemistry intricacies.
WRITE TO US
© 2024. All rights reserved.
