Chemical Buffer Systems & pH
Chemical Buffer Systems (Immediate Response):
Buffer systems provide the first and most immediate defense against pH changes, acting within seconds. These are weak acid-conjugate base pairs that resist changes in pH by absorbing excess hydrogen or hydroxyl ions.
These are three types:
1. Bicarbonate Buffer System
2. Phosphate Buffer System
3. Protein Buffer System
1. Bicarbonate Buffer System
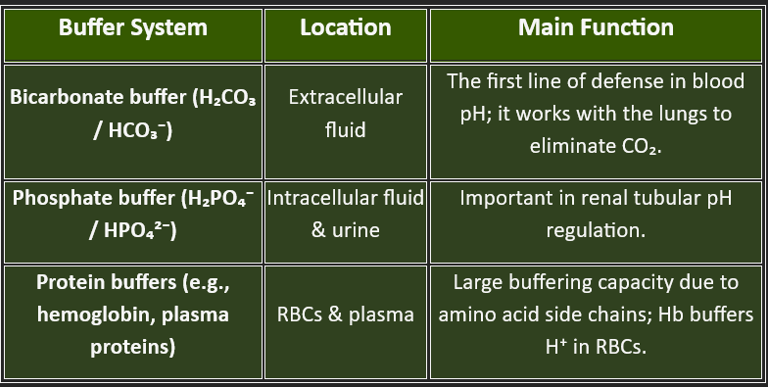
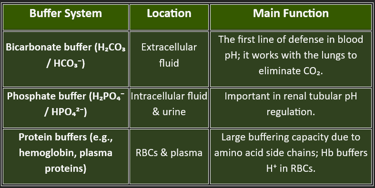
The bicarbonate-carbonic acid buffer system is the most important extracellular buffer.
It consists of:
Carbonic acid (H₂CO₃) - weak acid
Bicarbonate ion (HCO₃⁻) - conjugate base
The system operates according to the equilibrium:
CO₂ + H₂O ⇌ H₂CO₃ ⇌ H⁺ + HCO₃⁻
This buffer system maintains a 20:1 ratio of bicarbonate to carbonic acid in normal conditions, making it highly effective at buffering acids (which are more commonly produced by metabolism).
2. Phosphate Buffer System
The phosphate buffer system operates primarily in intracellular fluid and consists of:
Dihydrogen phosphate (H₂PO₄⁻) - hydrogen ion donor (acid)
Mono-hydrogen phosphate (HPO₄²⁻) - hydrogen ion acceptor (base)
3. Protein Buffer System
Proteins, including hemoglobin and plasma proteins like albumin, serve as important buffers. Amino acid side chains with exposed carboxyl groups (-COOH) act as weak acids, while amine groups (-NH₂) act as weak bases, allowing proteins to function as both acids and bases depending on the pH environment.

Definition: A buffer is a solution that resists changes in pH when small amounts of acid or base are added.
Components: A weak acid and its conjugate base (or a weak base and its conjugate acid).
Function in the body: Maintain a stable pH by binding free H⁺ when pH falls (acidic) or releasing H⁺ when pH rises (alkaline).
Examples in the human body:
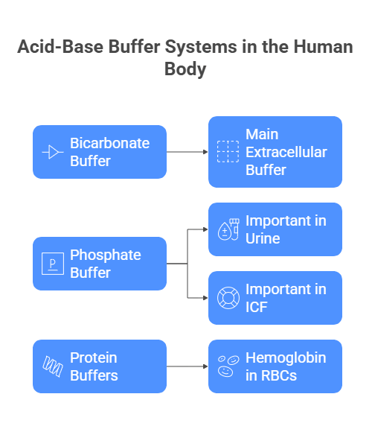
Bicarbonate buffer (H₂CO₃ / HCO₃⁻) – main extracellular buffer.
Phosphate buffer (H₂PO₄⁻ / HPO₄²⁻) – important in urine and ICF.
Protein buffers – especially hemoglobin in RBCs.
Significance of Buffers in Human
Think of buffers as your body’s pH shock absorbers. Every second, metabolism produces acids (e.g., CO₂ from respiration, lactic acid from muscles, ketone bodies in fasting) and bases. Without regulation, blood pH would swing wildly — and even small shifts can disrupt life.
Why buffers matter:
Prevent sudden pH changes: Immediate defense against acid or base load.
Protect enzyme function: Most enzymes work optimally at physiological pH (~7.4).
Maintain structural integrity of proteins: pH changes can denature proteins.
Support oxygen transport: Hemoglobin’s affinity for oxygen is pH-dependent (Bohr effect).
Aid in metabolic stability: Keep reactions running at the right rate.
Functions of Buffers
Buffers do not eliminate acids or bases — they neutralize them until the respiratory and renal systems can finish the job.
Carbonic anhydrase (in RBCs, renal tubular cells) rapidly interconverts CO₂ + H₂O ⇄ to H₂CO₃ ⇄ , H⁺ + HCO₃⁻.
Kidneys: Proximal tubule reabsorbs ~85% filtered HCO₃⁻; distal α-intercalated cells secrete H⁺ (H⁺-ATPase, H/K pump) and regenerate HCO₃⁻. Ammonium (NH₄⁺) excretion is critical for removing acid and creating new HCO₃⁻.
Immediate defense against pH change:
Acts within seconds to resist sudden changes in hydrogen ion concentration when acids or bases are added to body fluids.
Maintains physiological pH:
It keeps arterial blood pH between 7.35 and 7.45, which is essential for enzyme function and metabolic processes.
Prevents large shifts in [H⁺]:
Minimizes drastic fluctuations that could cause acidosis (<7.35) or alkalosis (>7.45).
Facilitates CO₂ transport:
Bicarbonate buffer plays a major role in converting CO₂ (from tissues) into bicarbonate (HCO₃⁻) for transport to the lungs, and vice versa.
Works with the respiratory and renal systems:
Buffers buy time for the lungs and kidneys to make longer-term adjustments in acid–base balance.
Maintains intracellular and extracellular stability:
Different buffers dominate in different compartments:
Bicarbonate buffer – extracellular fluid (ECF).
Phosphate buffer – intracellular fluid (ICF) and urine.
Protein buffers – both ICF & ECF (e.g., hemoglobin in RBCs).
Supports metabolic and cellular activity:
A stable pH is essential for the proper function of metabolic pathways, hormone activity, and nerve conduction.
pH of the human body
pH – Definition
pH is the negative logarithm of hydrogen ion concentration: pH=−log10[H+]
and it helps to find if the environment/solution is alkaline or acidic.
Normal pH in the Human Body
Arterial blood: 7.35 – 7.45 → slightly alkaline.
Venous blood: ~7.32 (slightly more acidic due to CO₂ from tissues).
Most intracellular fluids: ~7.0 – 7.2.
Gastric juice: ~1–2 (very acidic).
Urine: 4.5 – 8.0 (variable to maintain balance).
Significance of pH in the Human Body
Enzyme activity:
Most enzymes function best at physiological pH (~7.4).
Even a small deviation can slow or stop biochemical reactions.
Protein structure:
Changes in pH can alter protein folding (denaturation).
Electrolyte distribution:
pH affects the ionization of electrolytes and membrane potentials.
Oxygen transport:
Hemoglobin’s affinity for O₂ is pH-dependent (Bohr effect).
Metabolic stability:
Many pathways are pH-sensitive; unstable pH disrupts homeostasis.
Clinical Importance
Acidosis: pH < 7.35
→ Causes: respiratory depression, kidney failure, uncontrolled diabetes (ketoacidosis), shock.
→ Effects: CNS depression, confusion, coma, death if < 6.8.Alkalosis: pH > 7.45
→ Causes: hyperventilation, excessive vomiting, and diuretics.
→ Effects: muscle twitching, seizures, arrhythmias, death if > 7.8.Blood gas analysis:
pH measurement is part of the arterial blood gas (ABG) test, crucial in the ICU, surgery, and emergency care.
Drug action & absorption:
pH affects ionization of drugs (Henderson–Hasselbalch principle), influencing absorption and excretion.
BLOG
Join us to explore medical biochemistry intricacies.
WRITE TO US
© 2024. All rights reserved.
