Water and Electrolyte Balance
“Water and electrolytes are not just background molecules—they’re active regulators of life at every cellular level.”
Understanding their roles is fundamental for interpreting dehydration, edema, renal function, acid-base disorders, and electrolyte imbalances in both health and disease.
💧 Water and Electrolyte Balance: The Foundation of Fluid Homeostasis
💦 I. Water-Why It Matters 💦
Maintaining water and electrolyte balance is a basic but critical function of the human body. Every organ system—especially the kidneys, gastrointestinal tract, endocrine system, and cardiovascular system—works together to regulate this balance. Disruptions in fluid or electrolyte status can have serious consequences, particularly in critically ill patients.
Hence, understanding this topic helps you interpret IV fluids, assess hydration, and diagnose conditions like dehydration, shock, hyponatremia, or hyperkalemia.
I. Body Water: Distribution and Function
💧 Total Body Water (TBW)
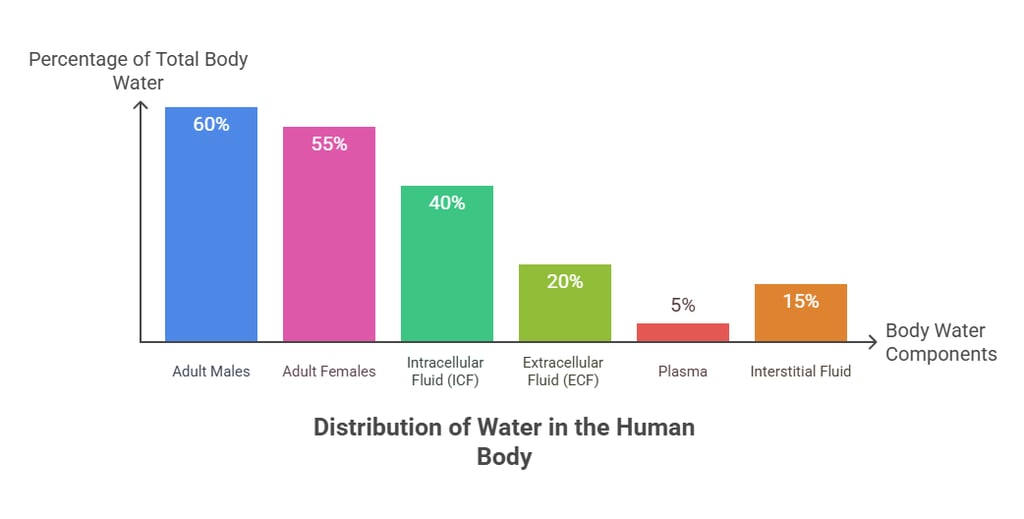
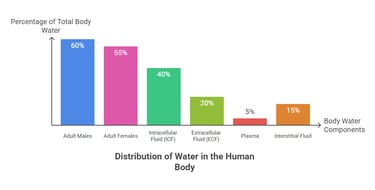
Accounts for ~60% of total body weight
~45–55% in women (higher fat content)
~65–75% in infants (less fat, more ECF)
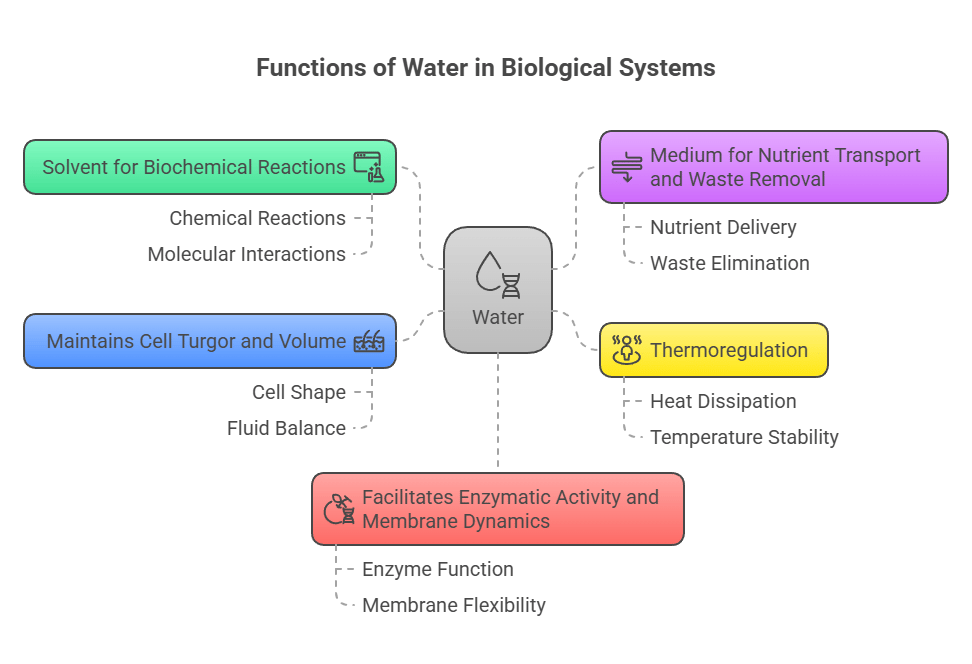
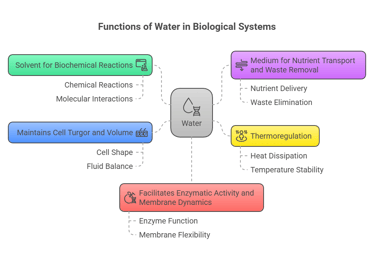
🧠 Functions of Water
Solvent for biochemical reactions
Medium for nutrient transport and waste removal
Thermoregulation
Maintains cell turgor and volume
Facilitates enzymatic activity and membrane dynamics
⚖️ Regulation of Water Balance
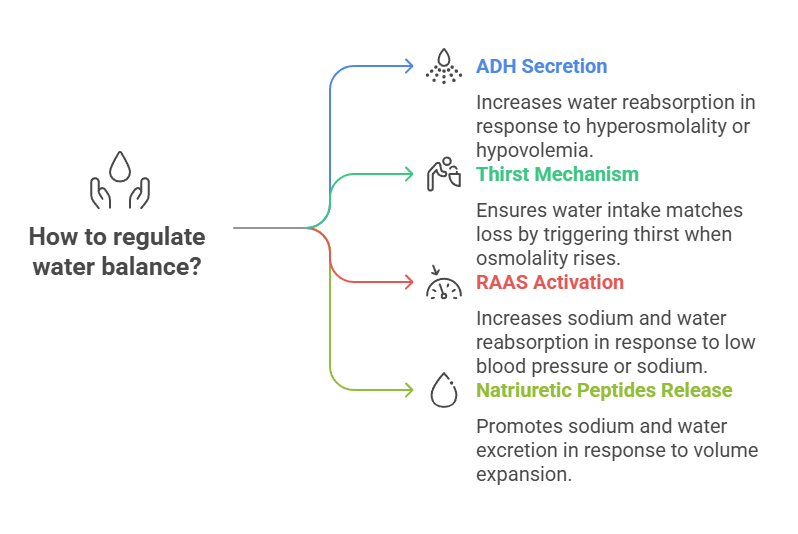
The body maintains fluid volume mainly through osmoregulation and volume regulation.
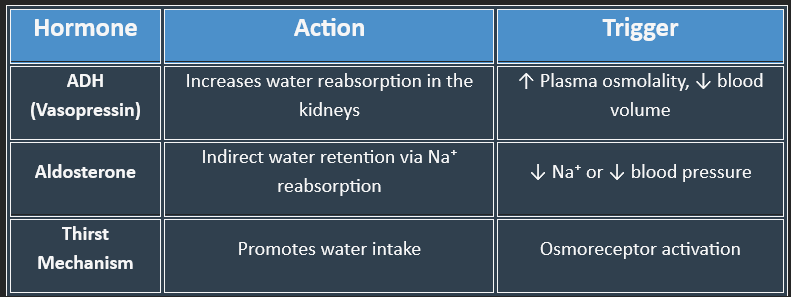
1. Antidiuretic Hormone (ADH) / Vasopressin
Secreted by the posterior pituitary in response to hyperosmolality or hypovolemia
Increases water reabsorption in the collecting ducts of the nephron
2. Thirst Mechanism
Triggered by osmoreceptors in the hypothalamus when plasma osmolality rises
Ensures water intake matches loss
3. Renin-Angiotensin-Aldosterone System (RAAS)
Activated in response to low blood pressure or sodium
Aldosterone increases sodium (and water) reabsorption in the distal tubule
4. Natriuretic Peptides (ANP, BNP)
Released by the heart in response to volume expansion
Promote sodium and water excretion
❗ Clinical Importance
Dehydration: Dry mucosa, hypotension, confusion
Overhydration: Edema, hyponatremia, seizures

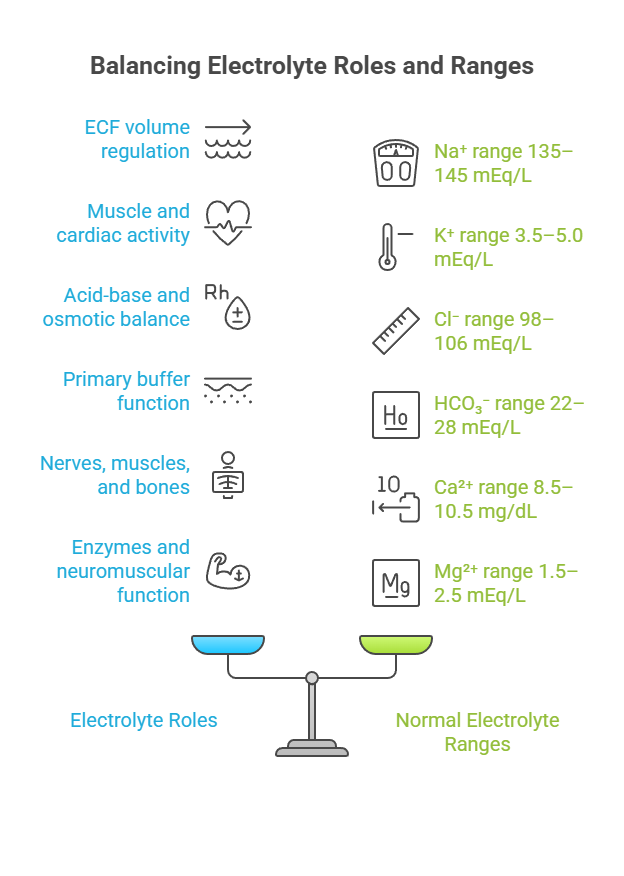
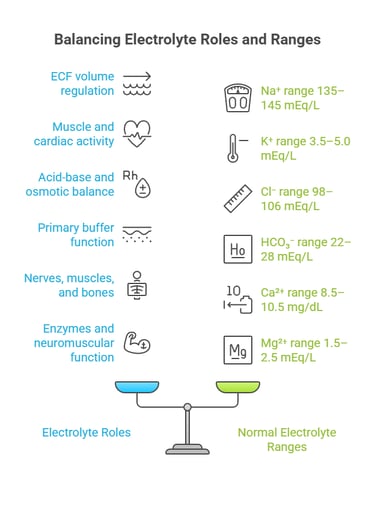
⚡ II. Electrolytes: The Ionic Players
Electrolytes are ions that help maintain fluid balance, membrane potential, and acid-base stability.
Electrolytes are ions that conduct electricity in solution and are essential for:
Osmotic balance
Nerve impulse transmission
Muscle contraction
Acid-base balance
Enzyme co-factors
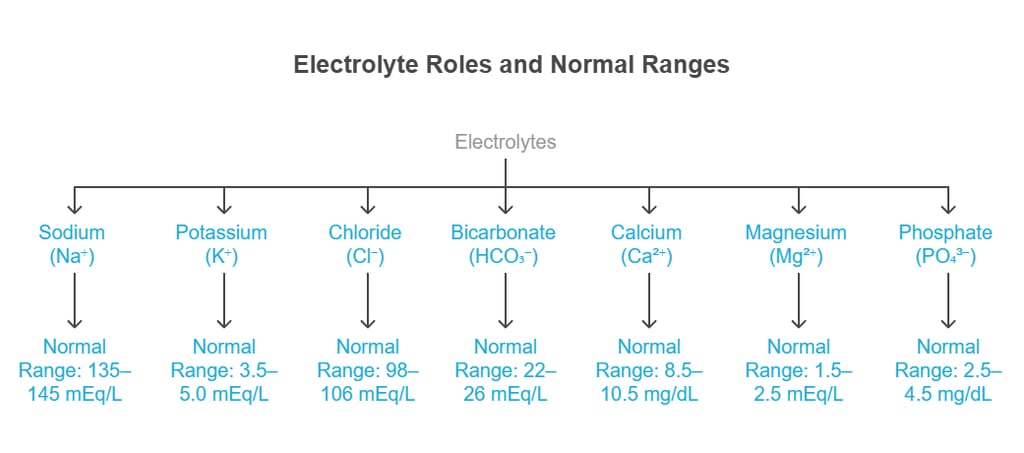
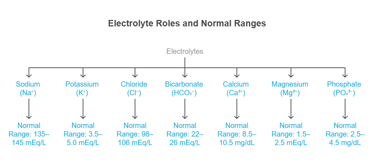
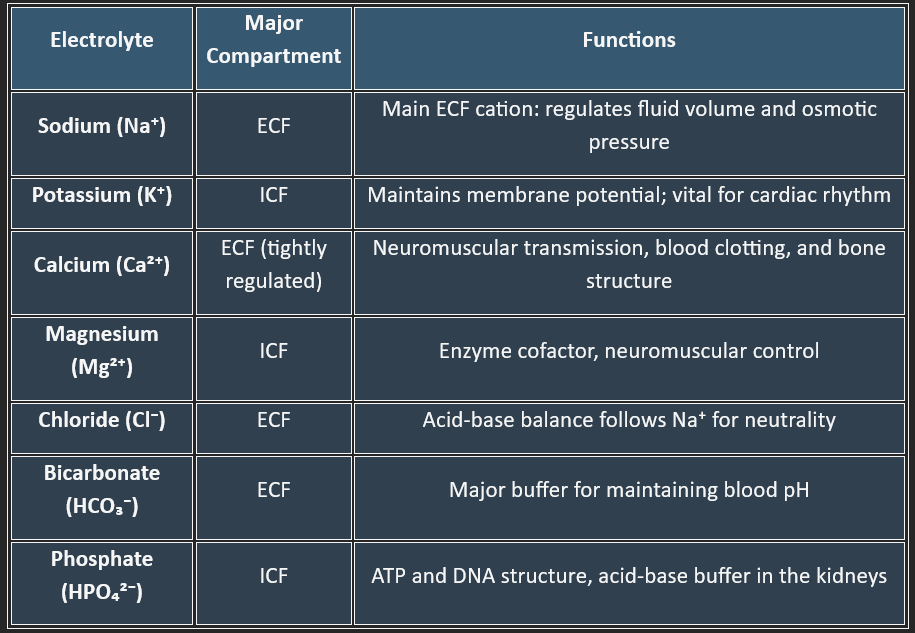

Major Electrolytes & Their Distribution


Regulation of Electrolyte Balance
Kidneys are the primary site for electrolyte regulation.


⚕️ Clinical Correlations
Loss of water > loss of electrolytes
Causes: diarrhea, vomiting, fever, burns
Signs: dry mucosa, hypotension, tachycardia, sunken eyes
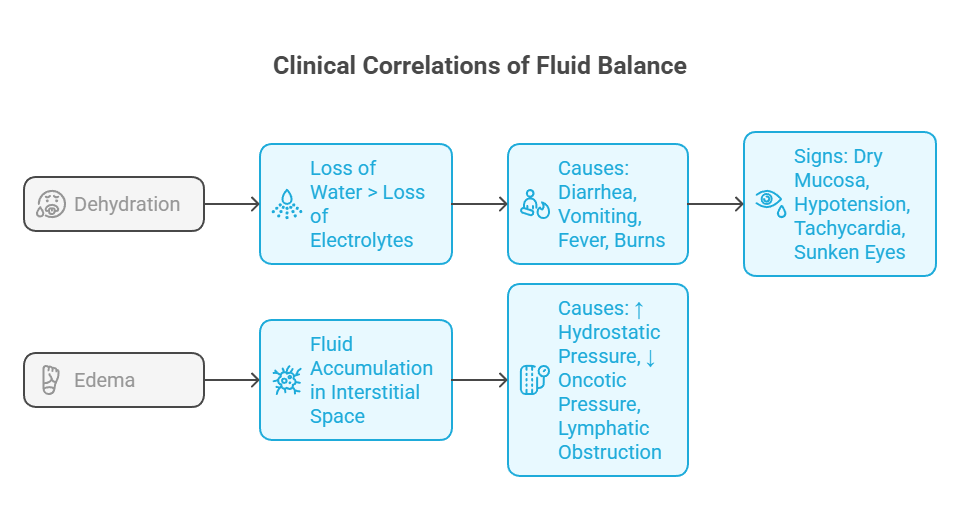
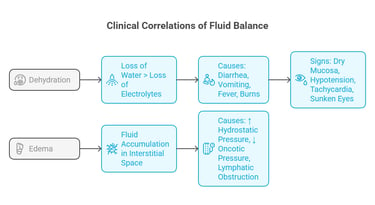
Fluid accumulation in the interstitial space
Causes: ↑ hydrostatic pressure, ↓ oncotic pressure, lymphatic obstruction
🥵 1. Dehydration
⚡ 3. Electrolyte Imbalances
💦 2. Edema
➤ Dehydration vs. Volume Depletion
Dehydration = loss of water only → ↑ plasma osmolality, hypernatremia
Volume depletion = loss of sodium and water → ↓ ECF volume, normal osmolality
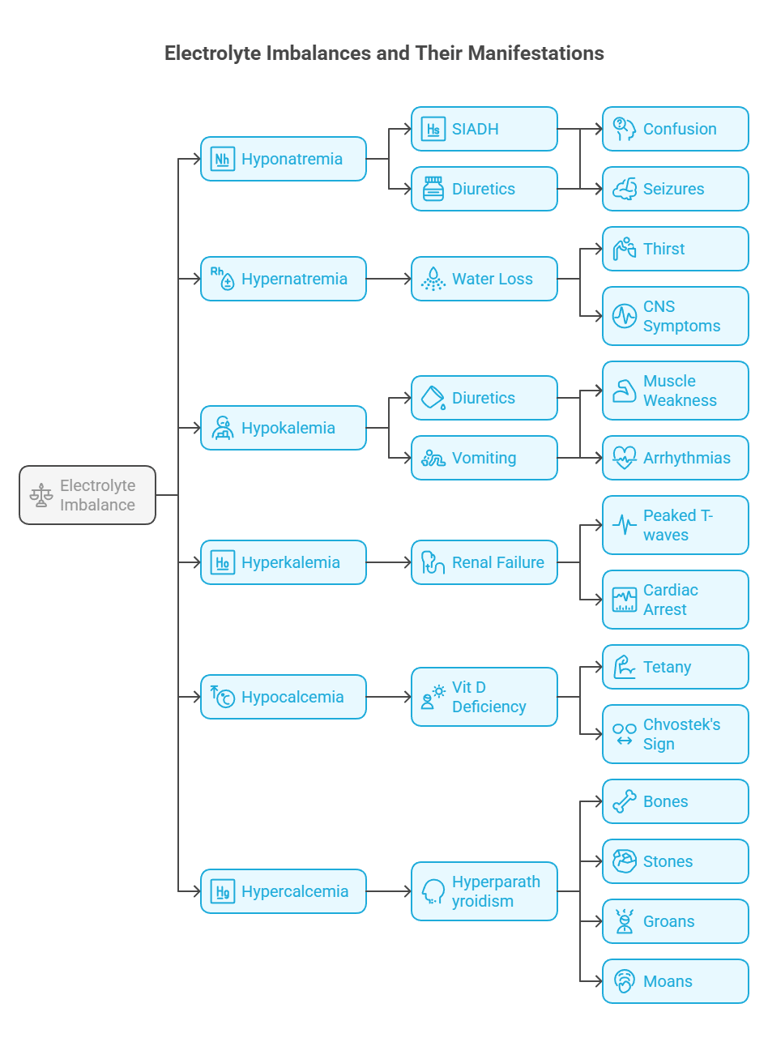
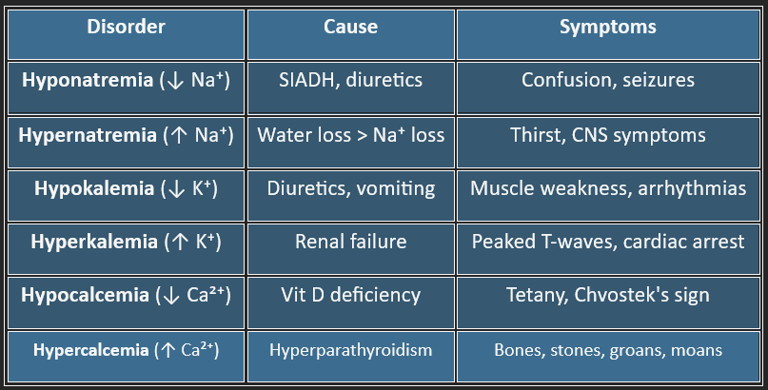
➤ Hyponatremia (Na⁺ < 135 mEq/L)
Causes: SIADH, fluid overload (CHF, cirrhosis), adrenal insufficiency
Symptoms: Nausea, confusion, seizures
Always assess serum osmolality and volume status (hypo-, eu-, or hypervolemic)
➤ Hypernatremia (Na⁺ > 145 mEq/L)
Causes: Water loss (fever, diabetes insipidus), inadequate intake
Symptoms: Lethargy, irritability, coma
Replace free water slowly to avoid cerebral edema
🔹 Common Fluid and Electrolyte Disorders
➤ Hypokalemia (K⁺ < 3.5 mEq/L)
Causes: Diuretics, vomiting, diarrhea, insulin excess
Symptoms: Muscle weakness, cramps, arrhythmias
EKG: Flattened T waves, U waves
➤ Hyperkalemia (K⁺ > 5.0 mEq/L)
Causes: Renal failure, acidosis, tissue breakdown
Symptoms: Palpitations, muscle paralysis
EKG: Peaked T waves, wide QRS, sine wave in severe cases
Urgent treatment: Calcium gluconate (stabilizes myocardium), insulin + glucose (shifts K⁺ into cells), dialysis if needed


🔄 Transport Mechanisms
Smart Student Tips
Always correlate electrolyte levels with hydration status.
Think of sodium as volume and potassium as heart function.
Plasma osmolality ≈ 2 × Na⁺ + Glucose/18 + Urea/2.8
(Useful in assessing dehydration and hyponatremia causes)
🎯 Final Takeaways
Water and electrolytes are critical for every physiological process.
Homeostasis is tightly regulated via hormones and osmoreceptors.
Imbalances manifest as neurological, cardiovascular, and muscular dysfunctions.
Every clinician must know how to interpret, correct, and prevent fluid-electrolyte disturbances.
BLOG
Join us to explore medical biochemistry intricacies.
WRITE TO US
© 2024. All rights reserved.
