🧬 Molecular Biology🧬
Understanding Life at the Molecular Level
The branch of biology that focuses on the molecular basis of life, specifically, how biological processes are regulated at the level of DNA, RNA, and proteins.
🧬 Core Definition 🧬
Molecular biology is the study of the structure, function, and interactions of cellular molecules—especially nucleic acids (DNA & RNA) and proteins—that are involved in the storage, expression, and regulation of genetic information.
🧬 Key Concepts in Molecular Biology: -
The Central Dogma
Gene Expression
Molecular Genetics
Recombinant DNA Technology
Protein Synthesis & Function
2. Gene Expression:
The process by which genes are turned on or off, and how their information is converted into functional products (like enzymes or hormones).
3. Molecular Genetics:
Studies mutations, genetic disorders, and how traits are inherited at the molecular level.
1. The Central Dogma:
DNA → RNA → Protein
Explains how genetic information flows within a cell.
DNA replication: Making exact copies of DNA.
Transcription: DNA is used to make RNA.
Translation: RNA is used to synthesize proteins.
4. Recombinant DNA Technology:
Techniques like PCR, cloning, CRISPR, and genetic engineering are used in biotechnology and medicine.
5. Protein Synthesis & Function:
How proteins are built and how they fold, interact, and regulate cellular processes.
1. The Central Dogma
The Central Dogma of Molecular Biology: A Journey Every Medical Student Must Know
Have you ever wondered how a single fertilized egg develops into a human being with trillions of cells, each carrying out highly specialized functions? Or how a mutation in a single gene can cause a disease that changes someone’s life?
The answer to both lies in one of the most elegant principles of modern biology: the Central Dogma of Molecular Biology.
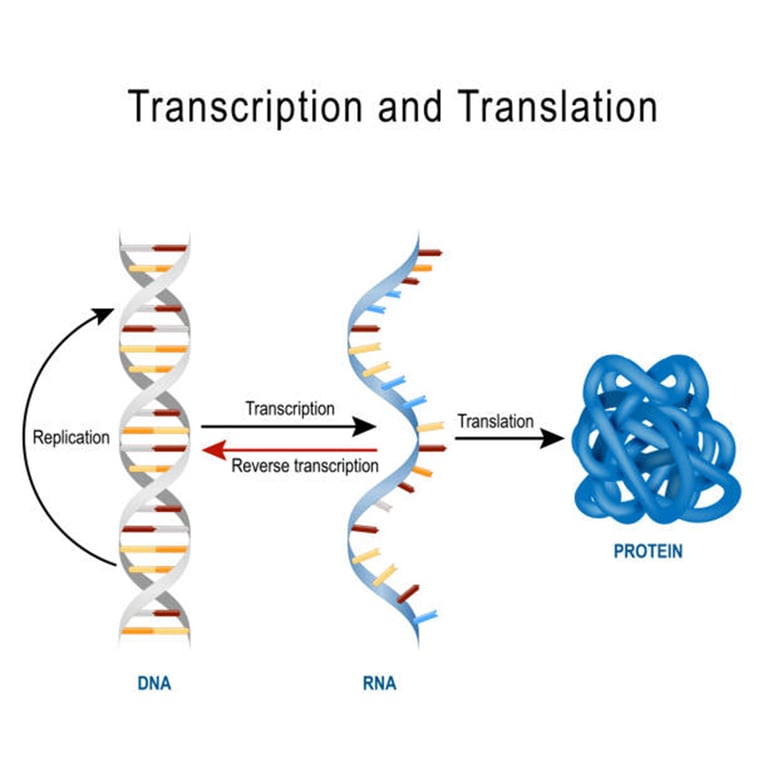
Definition: The Flow of Life’s Information
In simple terms, the central dogma explains how genetic information stored in DNA is used to create proteins — the molecules that actually do the work in our bodies.
It can be summarized as:
👉 DNA → RNA → Protein
DNA stores the genetic blueprint.
RNA acts as the messenger and translator.
Proteins are the final products that build structures, drive metabolism, carry oxygen, fight infections, and more.
👉Think of DNA as the textbook, RNA as the translator that makes notes, and proteins as the doctors, nurses, and paramedics who actually treat the patient. Without the proteins, the information in DNA is just sitting there, unused.
A Short History: From Mystery to Mastery
The story of the central dogma is as fascinating as the concept itself.
1869 – Friedrich Miescher isolates “nuclein” (later known as DNA).
1944 – Avery, MacLeod, and McCarty prove that DNA carries genetic information.
1953 – Watson and Crick, using Rosalind Franklin’s X-ray data, reveal the double helix structure of DNA.
1958 – Francis Crick formally proposes the “Central Dogma”: information flows from DNA to RNA to protein, and not in reverse.
1961–1965 – Scientists crack the genetic code, showing how RNA bases correspond to amino acids.
Modern twist – Today, we know it’s not always one-way. Retroviruses (like HIV) use reverse transcriptase to make DNA from RNA. Non-coding RNAs regulate gene expression. Yet, Crick’s central idea still holds as the foundation of molecular biology.
Why It Matters to Medical Students
This principle is seen in action every day:
In cancer, mutations in DNA can lead to uncontrolled protein production.
In genetic diseases, errors in the central dogma cause missing or defective proteins.
In viral infections, viruses hijack this flow of information to replicate.
In therapy, from mRNA vaccines to gene editing (CRISPR), modern medicine directly targets the central dogma to heal patients.
This is why the central dogma is taught early in the curriculum — it’s the cornerstone that connects basic biochemistry to patient care.
A Humane Perspective
At its core, the central dogma is not just about molecules. It’s about life’s continuity and fragility. Every heartbeat, every nerve impulse, every healing wound is made possible because cells follow this beautiful rule of life.
When something goes wrong in this process, disease results. When we understand it, we find cures.
So next time you’re memorizing transcription or translation, don’t think of it as dry theory.
Think of it as the language of life — the very process that makes you, you.
✨ Final Thought:
As medical students, embracing the central dogma is like learning the alphabet before writing poetry. Without it, nothing in biology makes sense; with it, you can begin to decode the miracles and mysteries of medicine.
2. Gene Expression
Imagine having the world’s greatest medical library, but all the books are locked away and unread. That’s what DNA would be if cells didn’t have a way to express it.
The central dogma tells us how information flows, but gene expression is about how the information is selected, read, and used — turning genetic instructions into real-life functions. It’s the reason why your heart cell beats, your neuron fires, and your liver cell detoxifies, even though every cell carries the same DNA.
Definition: What is Gene Expression?
In simple words, gene expression is the process by which the information stored in a gene (DNA) is used to synthesize a functional product (usually a protein, sometimes RNA).
It involves two main steps:
Transcription – copying DNA into messenger RNA (mRNA).
Translation – decoding mRNA into proteins.
But in medical terms, gene expression is more than that — it’s the control system of life. It decides which genes turn on, how strongly they are expressed, and when they are silenced.
Why It Matters in Medicine
Gene expression is not just a textbook topic — it’s a clinical reality:
Cancer – uncontrolled expression of growth genes leads to tumors.
Genetic diseases – failure to express critical proteins causes conditions like cystic fibrosis or thalassemia.
Infections – viruses manipulate host gene expression to survive.
Therapies – modern medicine uses siRNA, antisense therapy, and mRNA vaccines to directly alter gene expression.
This is why the DCI and MCI syllabi emphasize gene expression early on — because it is the bridge between biochemistry and disease management.
A Humane Perspective
Gene expression is, in many ways, the story of identity and specialization. Every cell in your body carries the same DNA, yet you have skin, muscle, nerves, and blood — because different genes are expressed in different contexts.
When this harmony is disrupted, disease follows. But when we understand it, we can design cures, predict outcomes, and save lives.
So, when you study transcription, splicing, or translation, remember — you’re not just learning about molecules. You’re learning how life chooses its path, one gene at a time.
✨ Final Thought:
Gene expression is life’s way of ensuring that information is not just stored but used wisely. For a medical student, mastering this concept is like learning the clinical reasoning behind symptoms — it connects the unseen molecular world to the visible realities of health and disease.
A Short History: From Mystery to Mechanism
The story of gene expression is a fascinating journey:
1869 – DNA is discovered by Friedrich Miescher.
1940s – Beadle and Tatum propose “one gene, one enzyme,” linking genes to proteins.
1953 – Watson & Crick describe DNA’s double helix.
1961 – Jacob and Monod propose the Operon Model, explaining gene regulation in bacteria.
1960s–70s – The genetic code is cracked, showing how RNA codons specify amino acids.
1977 – Discovery of introns and exons — proving that eukaryotic genes are not continuous, but edited before translation.
2000s – Human Genome Project reveals just 20,000–25,000 protein-coding genes, but complex regulation explains our diversity.
Modern era – Epigenetics, non-coding RNAs, and CRISPR revolutionize our understanding of gene control.
3. Molecular Genetics
Molecular Genetics in Molecular Biology: Decoding the Language of Life
If biochemistry is the chemistry of life, then molecular genetics is the storytelling of life. It explains how our genetic script — written in DNA — is read, edited, and passed on to future generations. For medical students, understanding molecular genetics isn’t just an academic requirement; it’s the foundation for diagnosing, treating, and even preventing genetic and acquired diseases.
Why It Matters in Medicine
For medical students, molecular genetics is not a theoretical chapter — it’s a clinical reality.
Inherited Disorders – Thalassemia, cystic fibrosis, and sickle-cell anemia are explained by mutations at the DNA level.
Cancer Genetics – Mutations in tumor suppressor genes or oncogenes drive malignancy.
Pharmacogenomics – Why a drug works in one patient but not another often depends on genetic variation.
Molecular Diagnostics – PCR, DNA sequencing, and microarrays help detect infections and genetic disorders with precision.
Gene Therapy & mRNA Vaccines – Turning our understanding of molecular genetics into life-saving interventions.
In short, from the lab bench to the bedside, molecular genetics guides modern medicine.
A Short History of Molecular Genetics
Milestones in the History of Molecular Genetics:
1860s – Gregor Mendel
The “Father of Genetics,” Mendel’s pea plant experiments revealed the basic laws of inheritance (though unnoticed at the time).1869 – Friedrich Miescher
Discovered “nuclein” (later identified as DNA) in white blood cells.1900 – Rediscovery of Mendel’s Work
Genetics officially gained recognition as a science.1910 – Thomas Hunt Morgan
Demonstrated that genes are carried on chromosomes (using fruit flies).1928 – Frederick Griffith
Discovered the “transforming principle” in bacteria, suggesting that some molecule carries genetic instructions.1944 – Avery, MacLeod & McCarty
Proved that DNA is the genetic material, not proteins.1952 – Hershey & Chase
Confirmed DNA as genetic material using bacteriophage experiments.1953 – Watson & Crick (with Franklin & Wilkins)
Revealed the double helix structure of DNA, revolutionizing biology.1960s – Cracking the Genetic Code
Nirenberg, Khorana, and colleagues deciphered how DNA triplets (codons) correspond to amino acids.1970s – Recombinant DNA Era
Cohen, Boyer, and others developed techniques to cut and paste DNA, launching genetic engineering.1980s–1990s – PCR & Sequencing
The invention of the Polymerase Chain Reaction (PCR) and automated sequencing accelerated genetic research.2003 – Human Genome Project
Completed the mapping of all 3 billion DNA base pairs, opening the age of genomics.Present Day – CRISPR & Beyond
Genome editing tools like CRISPR-Cas9 now allow precise editing of genes, offering potential cures for genetic diseases.
A Humane Perspective
Every strand of DNA carries not just chemical bases, but stories of ancestry, health, resilience, and vulnerability. Molecular genetics reminds us that while we all share 99.9% of our DNA, the tiny variations in genes give us our uniqueness — and sometimes our medical challenges.
For a medical student, learning molecular genetics is like learning the grammar of life. Once you understand how the language works, you can interpret the “mistakes” (mutations), predict their “meanings” (disease outcomes), and even suggest “corrections” (therapies).
✨ Final Thought:
Molecular genetics is not just about molecules and codes. It’s about life’s continuity, diversity, and vulnerability. By mastering this subject, you’re not just preparing for exams — you’re preparing to understand patients at their most fundamental level: their very blueprint of life.
4. Recombinant DNA Technology
Definition: What is Recombinant DNA Technology?
Recombinant DNA Technology is the process of cutting and joining DNA from different sources to create a new genetic combination that would not normally exist in nature.
In simpler words, scientists can “cut” a gene of interest (say, the insulin gene from humans), “paste” it into a carrier (like a bacterial plasmid), and then use that bacterium to produce insulin in bulk.
This technique forms the backbone of modern biotechnology, genetic engineering, and molecular medicine.
Why Recombinant DNA Technology Matters in Medicine:
For medical students, the importance of rDNA cannot be overstated. Here’s how it touches everyday medicine:
Insulin Production: No longer extracted from animal pancreas; bacteria make pure human insulin.
Vaccines: Safer, recombinant vaccines like Hepatitis B and HPV prevent millions of deaths.
Gene Therapy: Defective genes can be replaced with correct copies, offering hope for inherited disorders.
Monoclonal Antibodies: Targeted therapies for cancers, autoimmune diseases, and infections.
Molecular Diagnostics: PCR and DNA probes (built using rDNA concepts) detect pathogens quickly and accurately.
In short, rDNA technology transformed medicine from treating symptoms to targeting root genetic causes.
Imagine being able to take a gene from one organism, place it inside another, and make it work there — almost like moving an app from one device to another. This is exactly what Recombinant DNA Technology (rDNA Technology) allows us to do.
For medical students, this isn’t just a fancy lab trick. It’s the foundation for insulin production, vaccines, genetic therapies, and even cancer treatments.
👉In short, rDNA is where science meets imagination — and turns it into reality.
A Humane Perspective
Think about this: a child with Type 1 diabetes in the 1960s had limited options and a shorter life expectancy. Today, thanks to rDNA, insulin is abundant, affordable, and identical to human insulin. Similarly, recombinant vaccines have saved millions of children worldwide from preventable diseases.
Recombinant DNA isn’t just science; it’s a story of hope, equity, and the triumph of human creativity. It reminds us that biology isn’t static — we can rewrite parts of the story to heal and protect life.
✨ Final Thought:
Recombinant DNA Technology is one of the greatest revolutions in molecular biology. For medical students, mastering it is like holding the keys to modern medicine’s future — a future where genetic disorders, cancers, and infections can be tackled at the very roots of life itself.
A Short History of Recombinant DNA Technology
The journey of rDNA is both recent and revolutionary:
1953 – Watson & Crick: Discovery of DNA’s double-helix structure sets the stage.
1970 – Discovery of Restriction Enzymes: “Molecular scissors” that can cut DNA at specific sites.
1972 – Paul Berg: Creates the first recombinant DNA molecule, combining DNA from two different organisms.
1973 – Herbert Boyer & Stanley Cohen: Inserted foreign DNA into a plasmid and introduced it into E. coli — the first true example of gene cloning.
1978 – Genentech: Produces human insulin in bacteria using rDNA — a landmark in medicine.
1990s–2000s: rDNA used for vaccines (like Hepatitis B), monoclonal antibodies, and growth hormones.
Present day: The same principles power gene therapy, CRISPR-Cas9 editing, and personalized medicine.
5. Protein Synthesis & Function
Protein Synthesis & Function in Molecular Biology: Life’s Builders at Work
If DNA is the blueprint of life, then proteins are the workers, builders, and caretakers that bring the plan to reality. Every thought you think, every breath you take, every movement of your muscles — all depend on proteins.
Understanding how proteins are made (protein synthesis) and what they do (protein function) is essential for medical students because nearly every disease you study — from sickle cell anemia to cancer — has its roots in proteins gone wrong.
Protein Function: Why Are Proteins So Important?
Proteins are not just one thing — they are multifunctional machines of life. Their roles include:
Structural proteins: Collagen, keratin — giving strength to tissues, skin, hair, and bones.
Enzymes: Biological catalysts speeding up reactions (e.g., DNA polymerase, amylase).
Transport proteins: Hemoglobin carries oxygen, and membrane channels move ions.
Defense proteins: Antibodies protect against infections.
Signaling proteins: Hormones like insulin regulate body functions.
Motor proteins: Myosin and actin power muscle contraction.
In short, without proteins, life as we know it would simply stop.
Definition: What is Protein Synthesis?
Protein synthesis is the biological process by which cells build proteins, step by step, according to the instructions in DNA.
It’s often summarized as the “gene → protein” part of the central dogma of molecular biology.
This process involves two major stages:
Transcription – copying the DNA code into messenger RNA (mRNA).
Translation – reading that mRNA code at the ribosome and assembling amino acids into a functional protein.
Medical Importance of Protein Synthesis
For medical students, protein synthesis is not just theory — it explains clinical conditions and therapies:
Genetic Disorders: Mutations in DNA → faulty proteins (e.g., sickle cell anemia, cystic fibrosis).
Antibiotics: Many (like tetracycline and erythromycin) work by blocking bacterial protein synthesis, sparing human cells.
Cancer Biology: Overproduction of growth-related proteins drives tumor growth.
Pharmacology: Protein-based drugs (insulin, monoclonal antibodies) are lifesavers.
Neurobiology: Memory and learning depend on new protein synthesis in neurons.
Thus, protein synthesis connects molecular biology to bedside medicine.
A Humane Perspective
Imagine a newborn baby — their first cry, first heartbeat, first smile. Behind these simple moments is a silent orchestra of proteins being made tirelessly, second by second.
When protein synthesis falters, life struggles. But when science learns to guide or correct it, patients gain hope — whether it’s giving insulin to a diabetic child, monoclonal antibodies to a cancer patient, or enzyme replacement for rare genetic diseases.
Protein synthesis isn’t just a molecular process — it’s the language of life itself, one that medicine is steadily learning to read and rewrite.
✨ Final Thought:
Protein synthesis and protein function stand at the crossroads of biology and medicine. For medical students, understanding them is like unlocking the secret manual of the human body — the manual that explains health, disease, and the promise of new therapies.
A Short History of Protein Synthesis Discovery
The story of protein synthesis is a scientific detective tale:
1953 – DNA Structure: Watson and Crick’s double-helix discovery sparks the question: how does DNA make proteins?
1961 – Genetic Code Cracked: Marshall Nirenberg and Heinrich Matthaei deciphered the first codon, showing that RNA sequences correspond to specific amino acids.
1960s – Ribosome Role Clarified: Ribosomes were identified as the cell’s “protein factories.”
1970s–1980s: Detailed understanding of transcription and translation mechanisms emerged, aided by recombinant DNA techniques.
Modern Day: Structural biology and cryo-EM have revealed ribosomes and protein folding in stunning atomic detail.
What began as a mystery is now the foundation of genomics, biotechnology, and molecular medicine.
BLOG
Join us to explore medical biochemistry intricacies.
WRITE TO US
© 2024. All rights reserved.
