OVERVIEW OF MINERALS
Classification


Dietary Minerals:
Dietary minerals are inorganic elements essential for various physiological functions in the human body. They play a crucial role in maintaining health, supporting growth, and regulating biochemical processes.
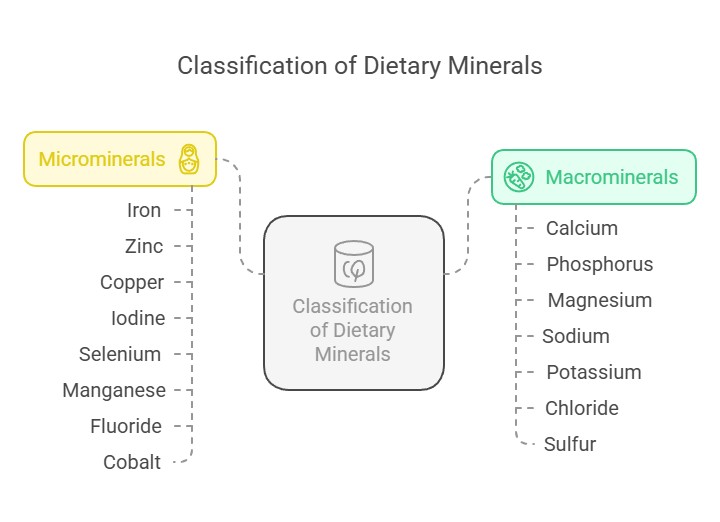
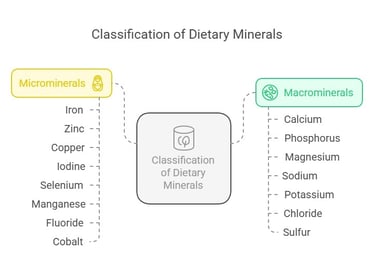
Classification of Dietary Minerals
Dietary minerals are classified into two main categories based on the quantity required by the body:
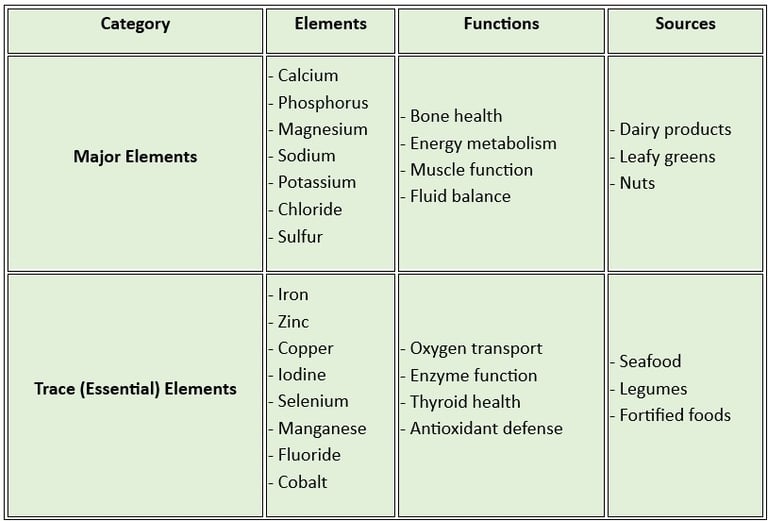
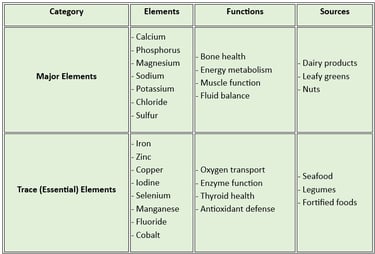
Macro minerals (Major Minerals): These are required in larger amounts (more than 100 mg/day). They include:
Calcium: Vital for bone health, muscle contraction, and blood clotting.
Phosphorus: Supports energy metabolism and bone mineralization.
Magnesium: Essential for enzyme activity and maintaining calcium- potassium balance.
Sodium regulates fluid balance and nerve function.
Potassium helps in muscle function and maintaining heart health.
Chloride: Aids in digestion and maintaining fluid balance.
Sulfur: Important for protein synthesis and detoxification.
Microminerals (Trace Minerals): These are required in smaller amounts (less than 100 mg/day). They include:
Iron: Crucial for oxygen transport in the blood.
Zinc supports immune function and wound healing.
Copper helps in iron metabolism and enzyme function.
Iodine: Necessary for thyroid hormone production.
Selenium Acts as an antioxidant and supports thyroid function.
Manganese: Involved in bone formation and enzyme activity.
Fluoride: Strengthens teeth and prevents dental caries.
Cobalt: A component of vitamin B12.
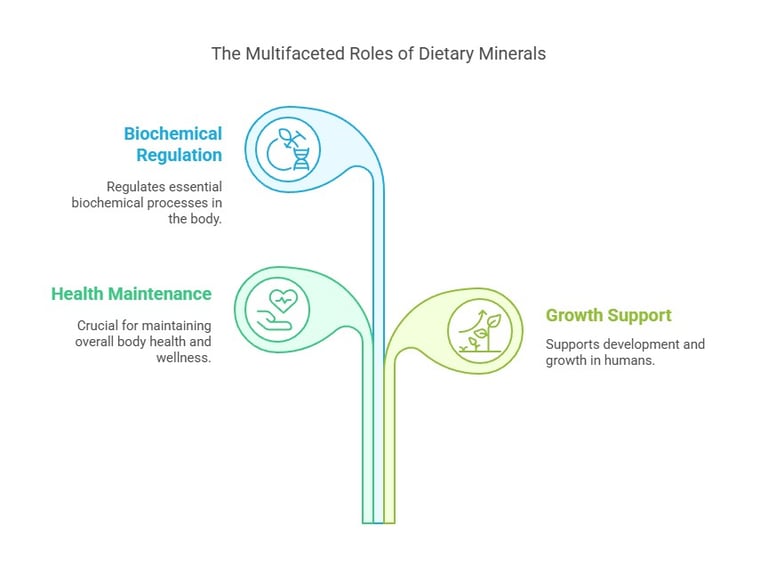
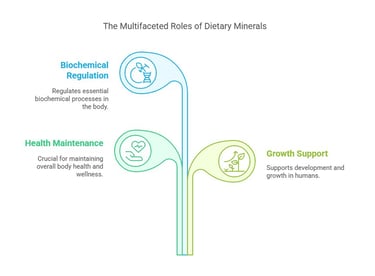
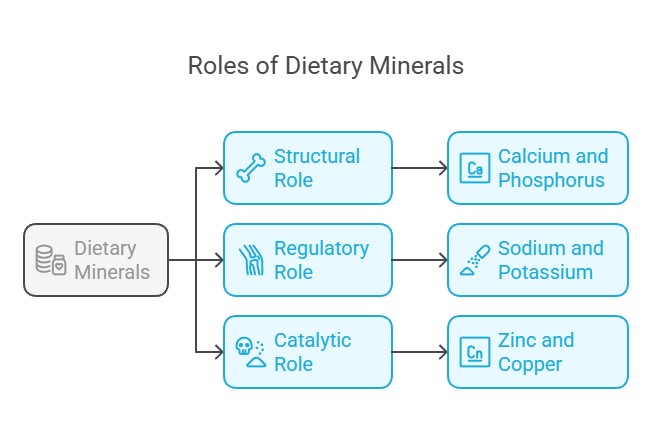
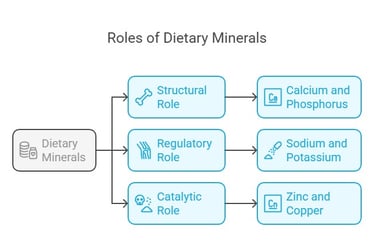
Key Functions of Dietary Minerals
Structural Role: Minerals like calcium and phosphorus are integral to bones and teeth.
Regulatory Role: Sodium and potassium regulate nerve impulses and muscle contractions.
Catalytic Role: Trace minerals like zinc and copper act as cofactors for enzymes.
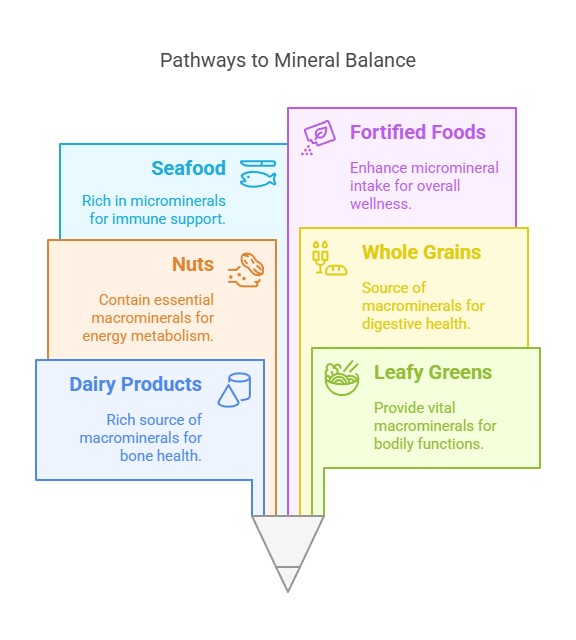
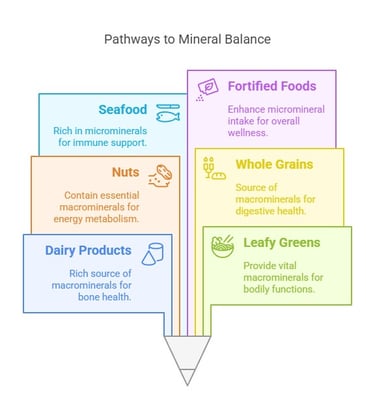
Sources of Dietary Minerals
Macro minerals: These are found in dairy products, leafy greens, nuts, and whole grains.
Microminerals: Present in seafood, meat, legumes, and fortified foods.
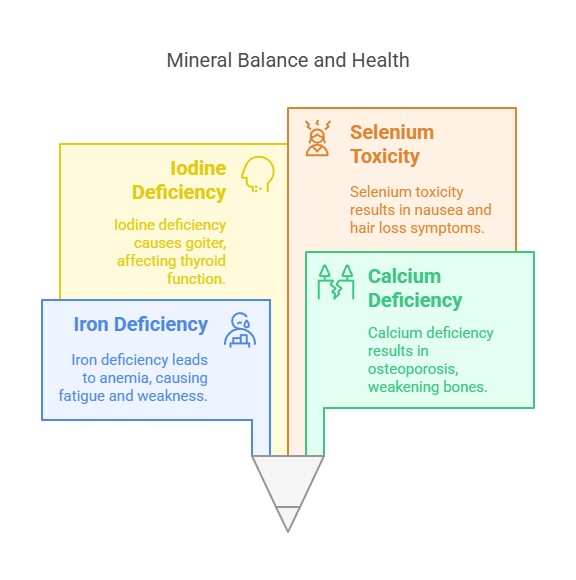
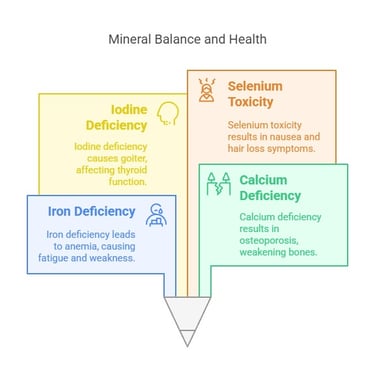
Deficiency and Toxicity
Deficiency: Lack of minerals can lead to conditions like anemia (iron deficiency), osteoporosis (calcium deficiency), and goiter (iodine deficiency).
Toxicity: Excessive intake can cause adverse effects, such as selenium toxicity, leading to nausea and hair loss.
QUICK RECAP: MINERALS
A clear table distinguishing essential trace elements and major elements
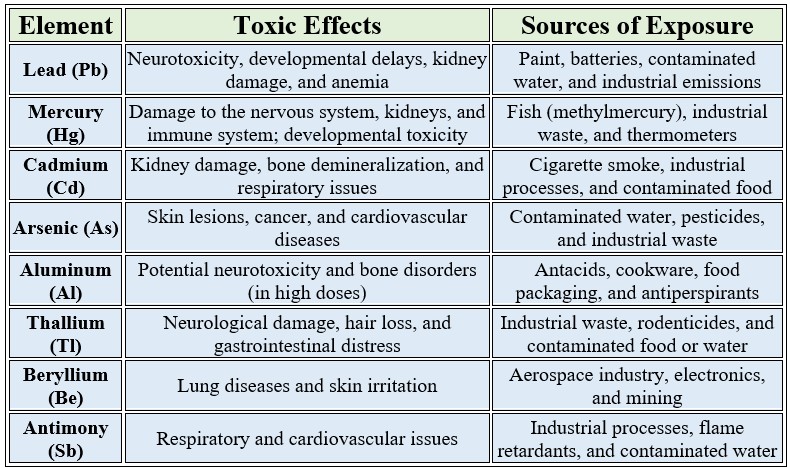
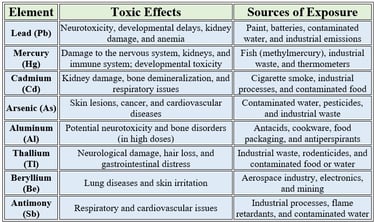
Toxic Trace Elements with No Biological Value
These elements are not required for any physiological function and can be harmful even in small amounts
Key Points to Note
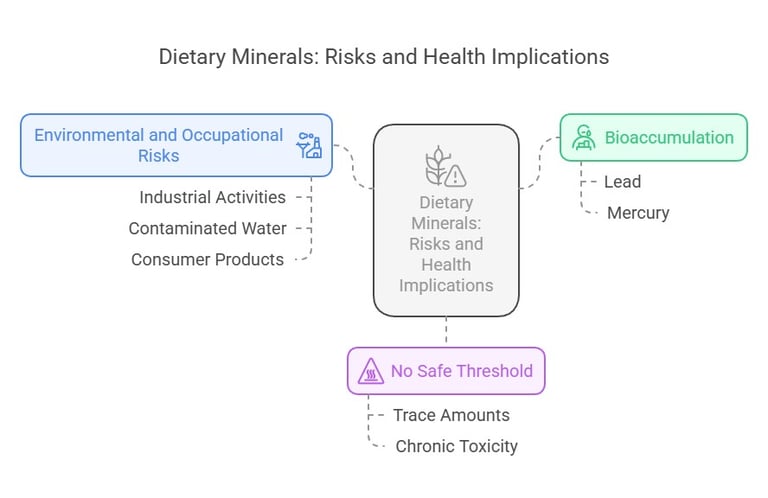
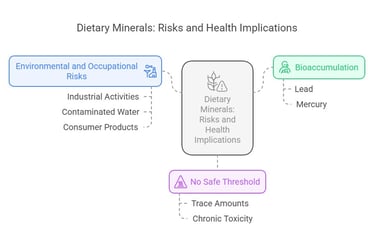
Bioaccumulation: Some of these elements, like lead and mercury, can accumulate in the body over time, leading to chronic toxicity.
No Safe Threshold: For many of these elements, there is no truly safe exposure level, as even trace amounts can be harmful.
Environmental and Occupational Risks: Exposure often occurs through industrial activities, contaminated water, or certain consumer products.
Calcium Overview
Absorption-Excretion-Regulation-Functions-Disorders
Calcium is the most abundant mineral in the human body, accounting for approximately 1-2% of adult body weight. It plays crucial roles in structural, regulatory, and enzymatic functions.
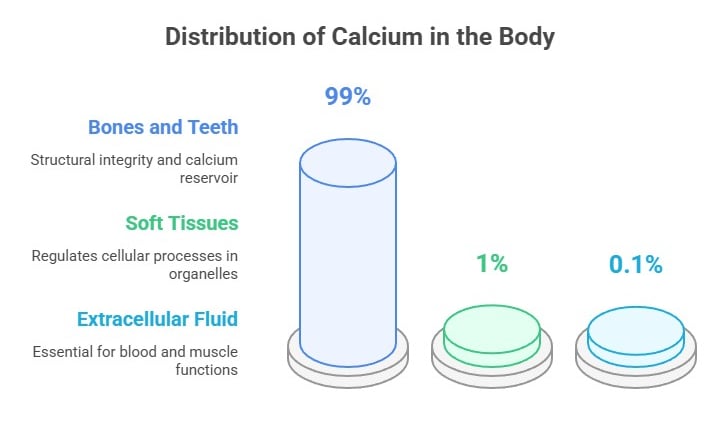
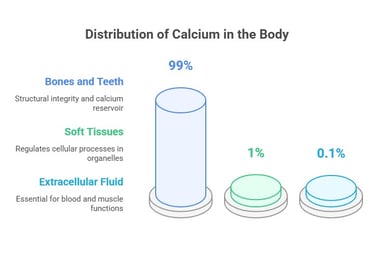
Calcium Distribution in the Body
Calcium is a vital mineral, and its distribution in the body reflects its diverse physiological roles:
Bones and Teeth (99% of total calcium):
Most calcium is stored in bones and teeth as hydroxyapatite crystals. This provides structural integrity and acts as a reservoir for maintaining calcium homeostasis.
Bones undergo constant remodeling—osteoclasts release calcium while osteoblasts deposit it during bone formation.
Soft Tissues (1%):
Calcium is found within cells in organelles like the endoplasmic reticulum and mitochondria, where it helps regulate various cellular processes.
Extracellular Fluid:
Approximately 0.1% of the total calcium exists in the bloodstream and interstitial fluids.
It is tightly regulated within a narrow range (8.5–10.2 mg/dL) to ensure proper physiological functions like blood clotting, nerve transmission, and muscle contraction.
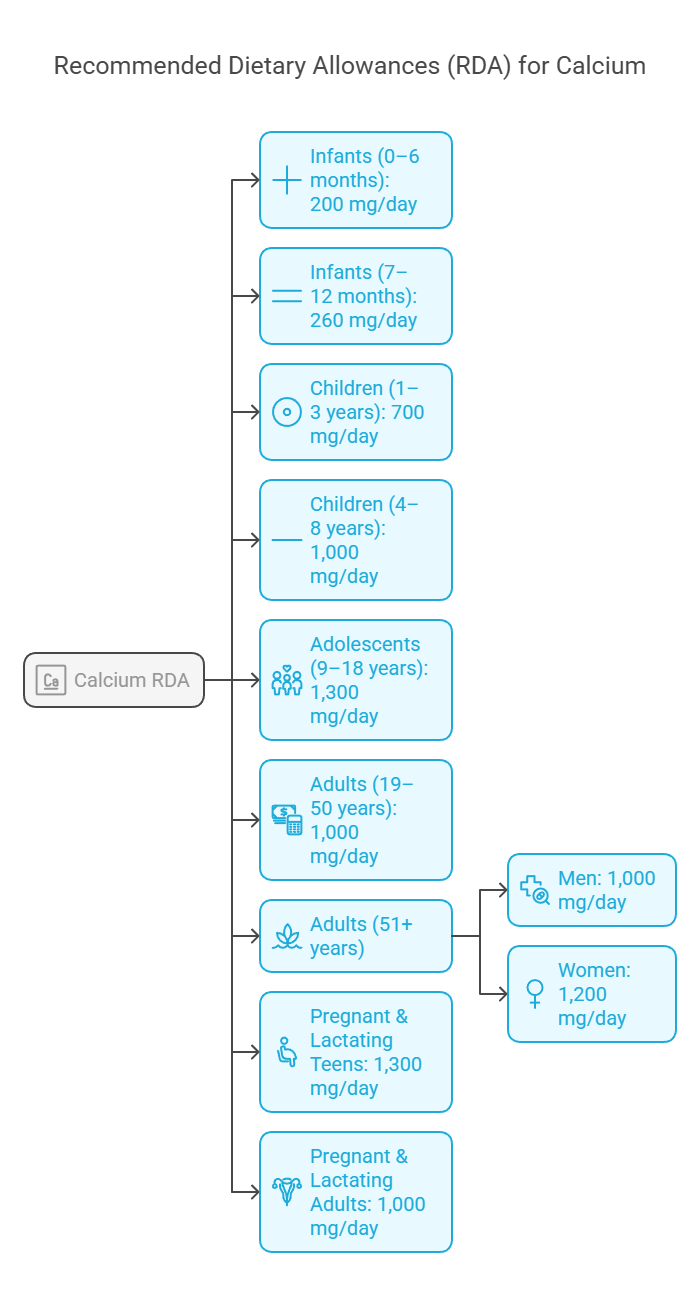
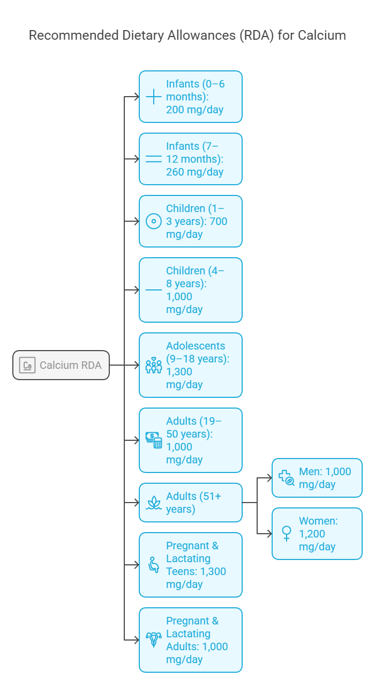
Calcium Requirement as per Recommended Dietary Allowance (RDA)
The Recommended Dietary Allowance (RDA) for calcium varies depending on age, sex, and life stage. A detailed breakdown based on guidelines provided by nutritional authorities like the National Institutes of Health (NIH) is in the table
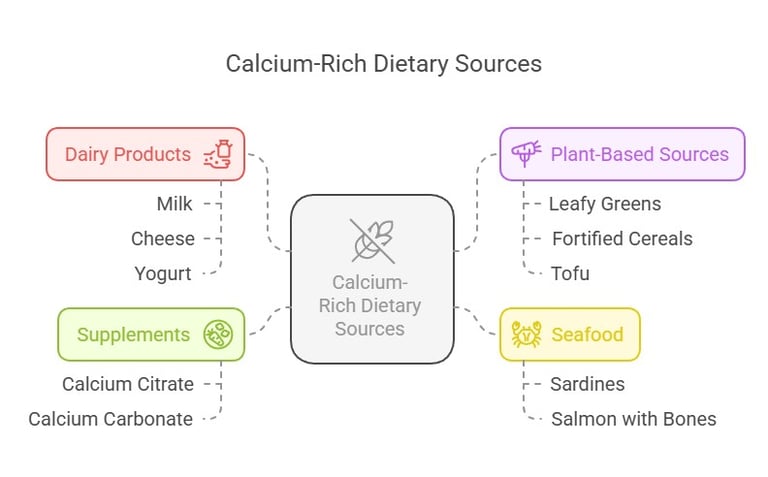
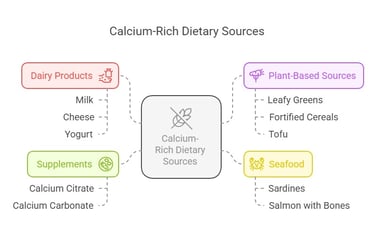
Calcium Sources
To meet RDA levels, calcium-rich dietary options include:
Dairy Products: Milk, cheese, and yogurt.
Plant-Based Sources: Leafy greens (like kale and spinach), fortified cereals, and tofu.
Seafood: Sardines and salmon (with bones).
Supplements: Calcium citrate and calcium carbonate, especially in cases of deficiency.
Clinical Importance of RDA
Deficiency Risks: Inadequate calcium intake leads to conditions like osteopenia, osteoporosis, and rickets.
Excess Risks: Over-supplementation may cause hypercalcemia, kidney stones, or vascular calcification.
Through awareness of calcium distribution and RDA requirements, learners can better appreciate its critical role in health and disease prevention.
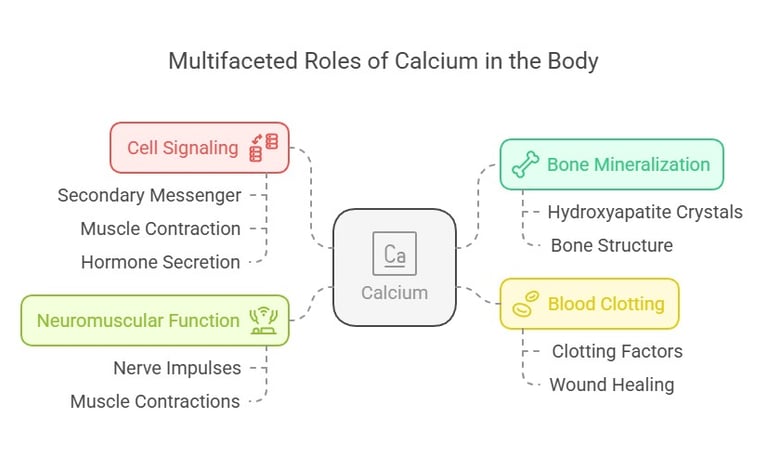
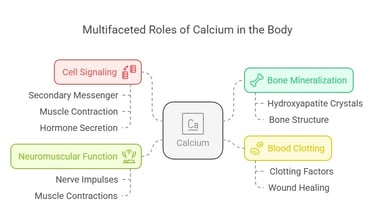
The Role of Calcium: A Brief Overview
Calcium isn’t just about strong bones—it’s a multitasking mineral at the heart of your body’s daily operations. Some of its key roles include:
Bone Mineralization: Calcium is the main ingredient in hydroxyapatite crystals that provide structure to bones and teeth.
Cell Signaling: It acts as a secondary messenger in pathways like muscle contraction and hormone secretion.
Blood Clotting: Calcium enables activation of clotting factors essential for wound healing.
Neuromuscular Function: It ensures proper functioning of nerve impulses and muscle contractions.
Absorption of Calcium
Calcium absorption is a meticulously regulated process that ensures the body maintains sufficient calcium levels for critical functions, including bone health, muscle contraction, and nerve signaling. Below is an engaging breakdown of calcium absorption.
Where Does Calcium Absorption Occur?
The primary site for calcium absorption is the small intestine, particularly the duodenum and jejunum, with minor contributions from the ileum. Here, calcium from dietary sources is absorbed into the bloodstream through two mechanisms:
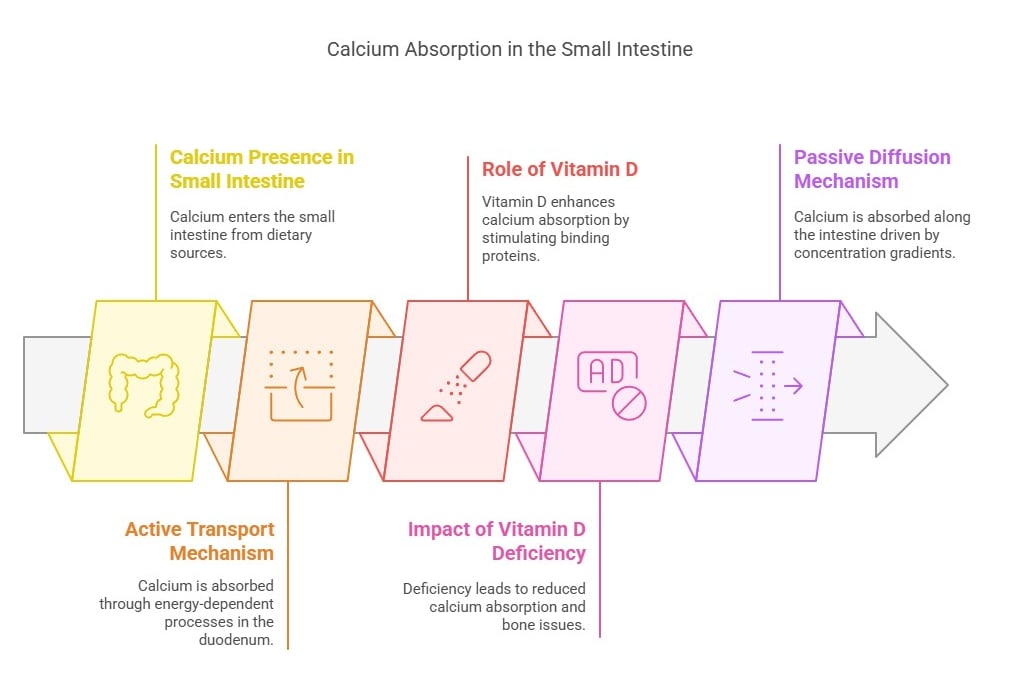
1. Active Transport (Requires energy):
Occurs in the duodenum and is especially efficient when calcium intake is low.
Role of Vitamin D: Active vitamin D (calcitriol) enhances calcium absorption by stimulating the synthesis of calcium-binding proteins in intestinal epithelial cells.
Clinical Connection: Vitamin D deficiency reduces active calcium absorption, leading to bone-softening diseases like rickets and osteomalacia.
2. Passive Diffusion (Does not require energy):
Occurs throughout the intestine and is driven by concentration gradients.
Effective when dietary calcium intake is high but is less regulated than active transport.
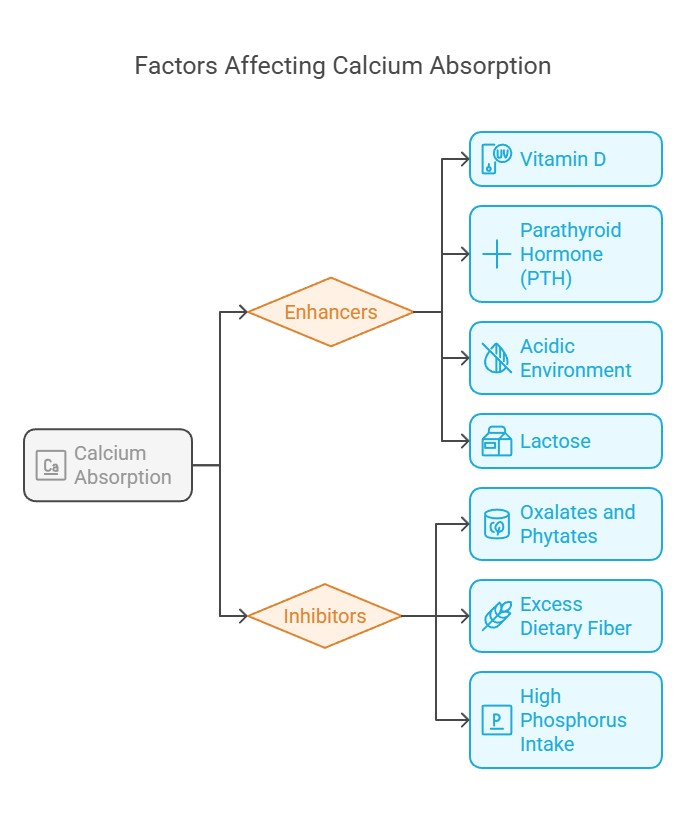
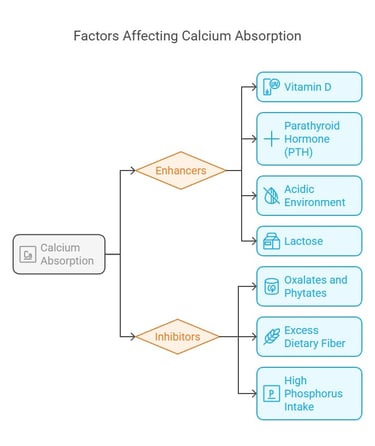
Factors Affecting Calcium Absorption
Several factors influence how well calcium is absorbed:
1. Enhancers:
a) Vitamin D: Stimulates calcium-binding proteins in intestinal cells.
b) Parathyroid Hormone (PTH): Indirectly boosts absorption by
activating vitamin D synthesis.
c)Acidic Environment: Promotes solubility of calcium salts, aiding
absorption.
d)Lactose: Found in dairy; enhances calcium uptake.
2. Inhibitors:
a) Oxalates and Phytates: Found in foods like spinach and grains,
they bind calcium, preventing absorption.
b) Excess Dietary Fiber Reduces calcium bioavailability by binding
to it.
c)High Phosphorus Intake: Competes with calcium for absorption.
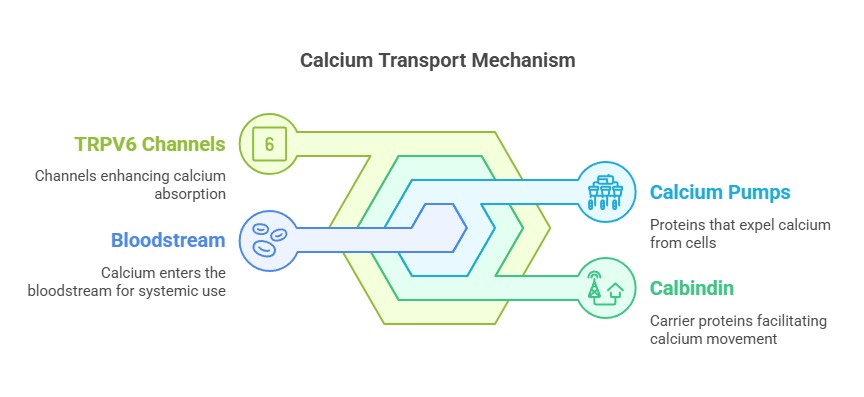
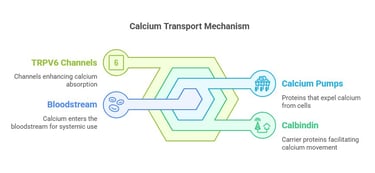
Pathways of Calcium Absorption
1. Vitamin D-Dependent Active Transport:
Vitamin D increases the expression of TRPV6 channels in intestinal epithelial cells, facilitating calcium entry.
Calcium is transported across the cell via proteins like calbindin and exits into the bloodstream through calcium pumps.
Pathways of Calcium Absorption
2. Passive Transport:
Calcium flows down its concentration gradient, entering intestinal cells without requiring energy or specific transporters.
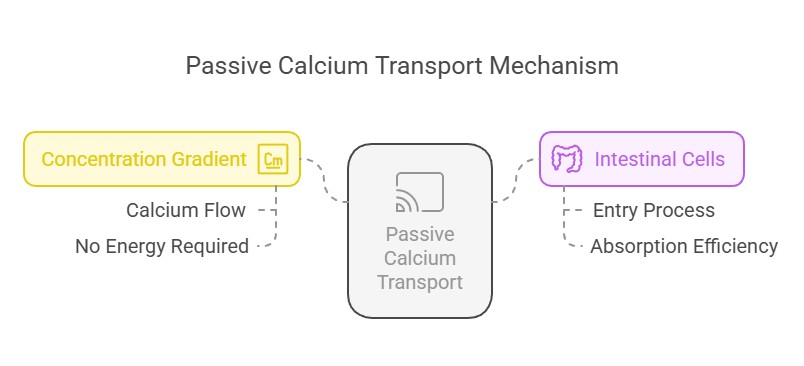
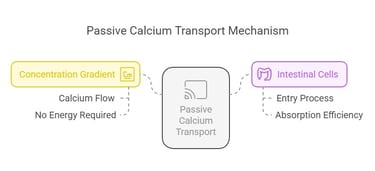
Clinical Correlations
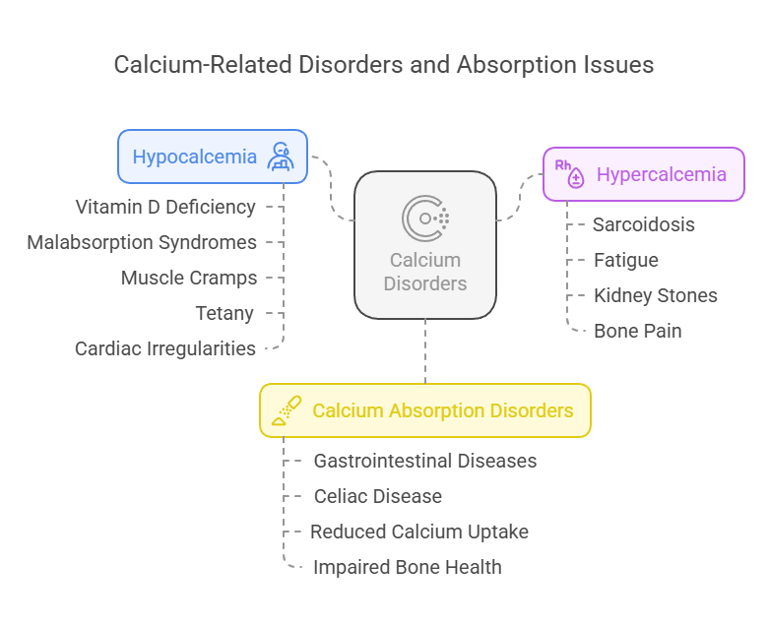
1. Hypocalcemia:
a) Low blood calcium levels often arise from reduced
intestinal absorption due to vitamin D deficiency or
malabsorption syndromes.
b) Symptoms include muscle cramps, tetany, and
cardiac irregularities.
2. Hypercalcemia:
a) Excess calcium absorption, as seen in conditions
like sarcoidosis (overproduction of vitamin D), can
lead to fatigue, kidney stones, and bone pain.
3. Calcium Absorption Disorders:
a) Malabsorption due to gastrointestinal diseases like
celiac disease reduces calcium uptake, impacting
bone health.
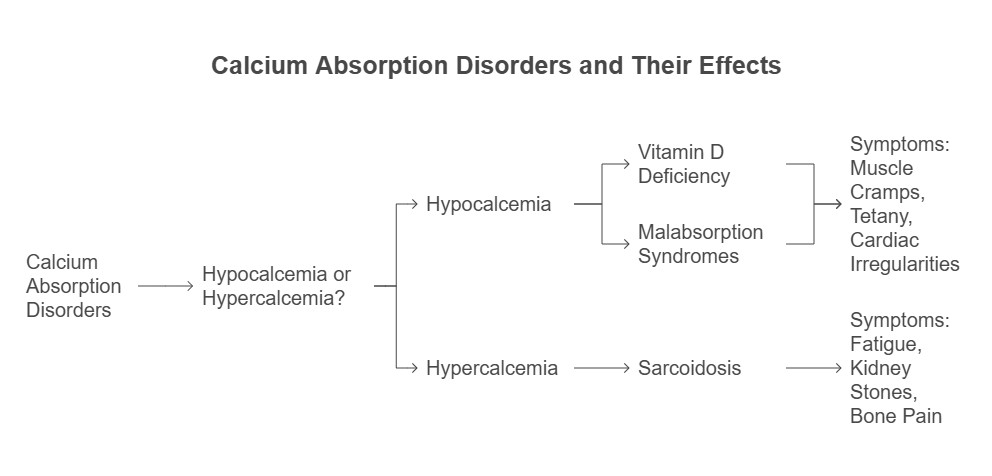
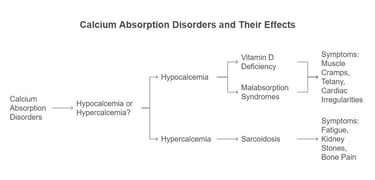
Calcium Absorption Disorders & their Effects
Phosphorus: An Essential Mineral
Body Distribution
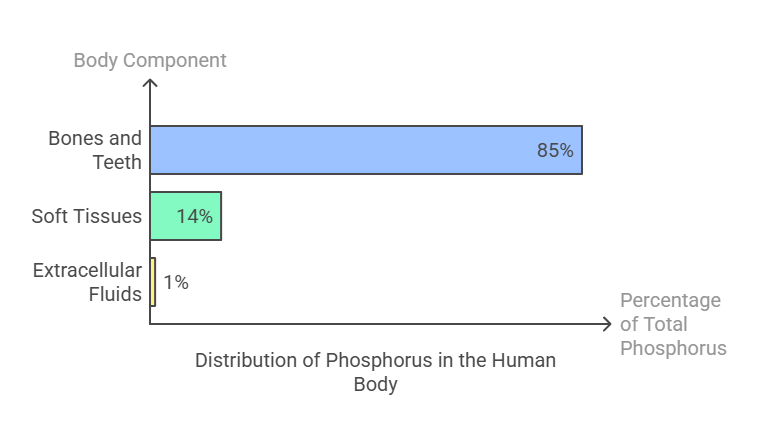
Phosphorus is the second most abundant mineral in the human body, accounting for approximately 1% of total body weight. Its distribution is as follows:
Approximately 85% of calcium is stored in bones and teeth as hydroxyapatite crystals, providing structural strength.
14% is found in soft tissues, including muscles and organs.
1% resides in extracellular fluids, where it plays a critical role in metabolic processes.
Biological Functions
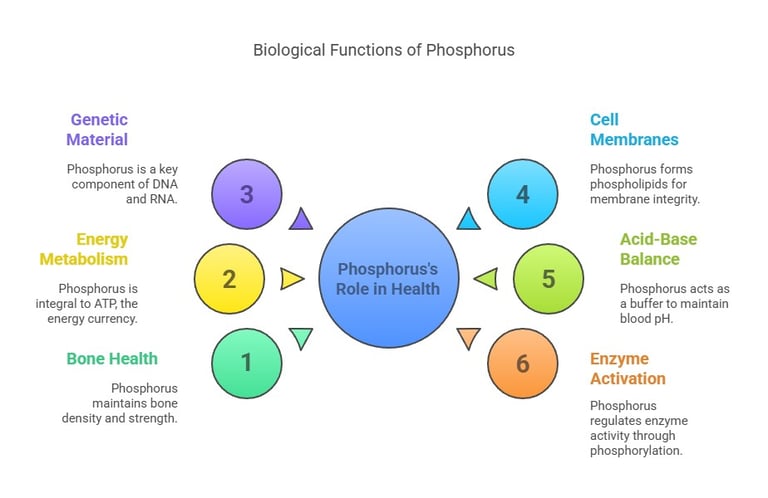
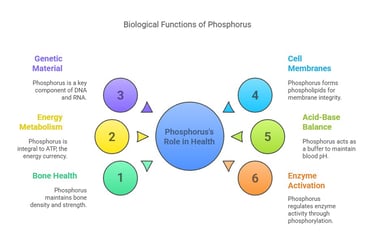
Phosphorus is indispensable for numerous physiological processes:
Bone and Teeth Health: Works with calcium to maintain bone density and strength.
Energy Metabolism: Integral to ATP (adenosine triphosphate), the energy currency of cells.
Genetic Material: A key component of DNA and RNA, essential for cell replication and protein synthesis.
Cell Membranes: Forms phospholipids, which are vital for cell membrane integrity.
Acid-Base Balance: Acts as a buffer to maintain blood pH.
Enzyme Activation: Regulates enzyme activity through phosphorylation.
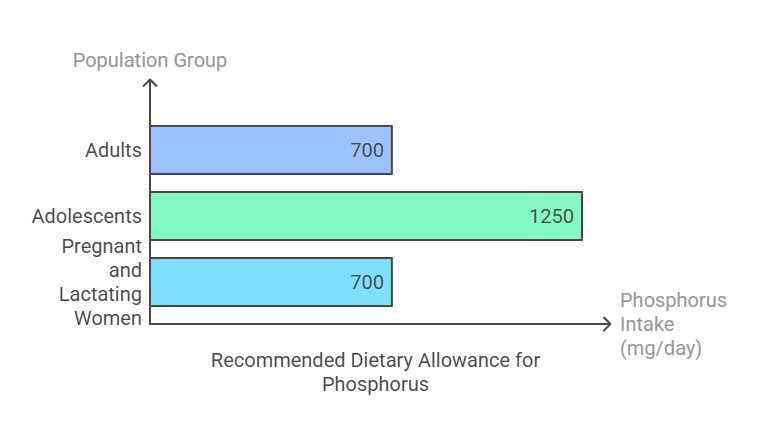
Dietary Requirement
The Recommended Dietary Allowance (RDA) for phosphorus varies by age and physiological state:
Adults (19+ years): 700 mg/day.
Adolescents (9–18 years): 1,250 mg/day (due to rapid growth).
Pregnant and lactating women: 700 mg/day. Excessive intake (above 4,000 mg/day for adults) can lead to adverse effects, especially in individuals with kidney issues.
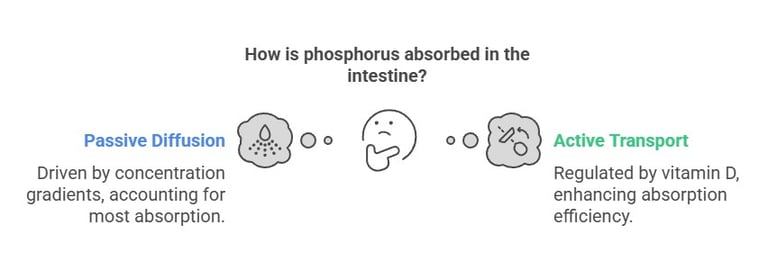
Intestinal Absorption
Phosphorus absorption occurs primarily in the small intestine through:
Passive Diffusion: Accounts for most absorption, driven by concentration gradients.
Active Transport: Regulated by vitamin D (1,25-dihydroxyvitamin D), which enhances absorption efficiency. Absorption rates range from 55% to 70%, depending on dietary sources and individual health.
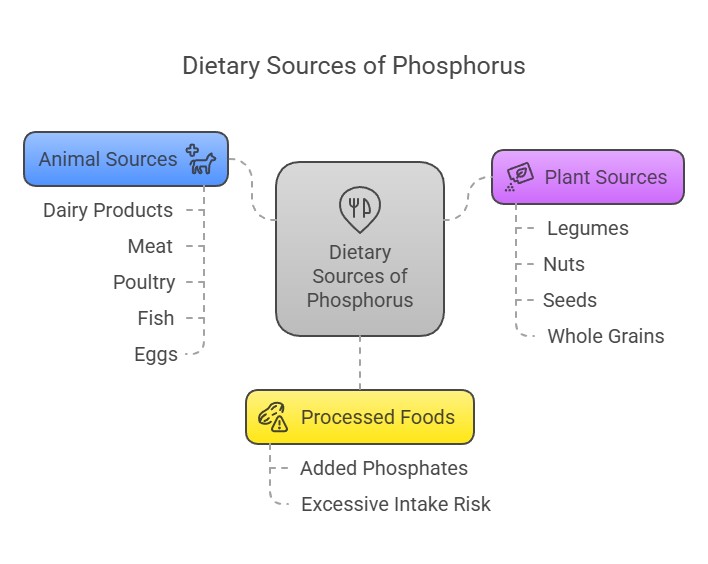
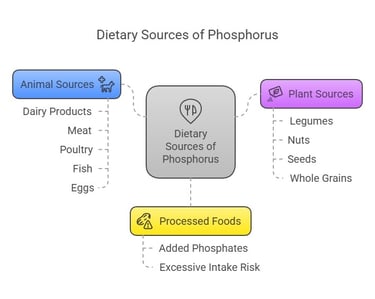
Dietary Sources
Phosphorus is abundant in a variety of foods:
Animal Sources: Dairy products, meat, poultry, fish, and eggs.
Plant Sources: Legumes, nuts, seeds, and whole grains (though plant-based phosphorus is less bioavailable due to phytates).
Processed Foods: Often contain added phosphates, which are highly absorbable but can contribute to excessive intake.
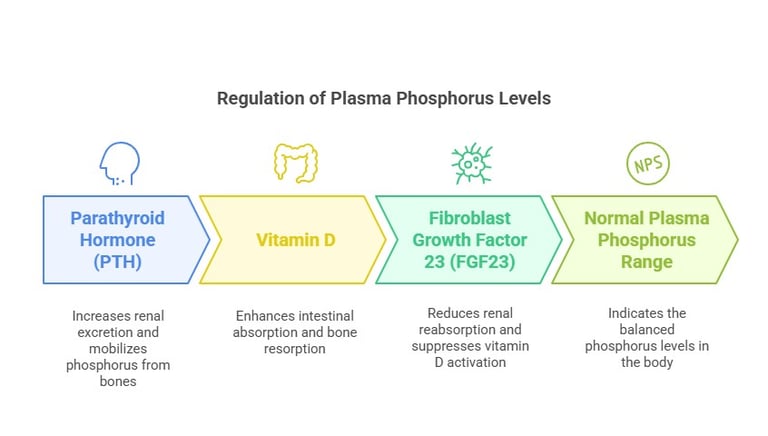
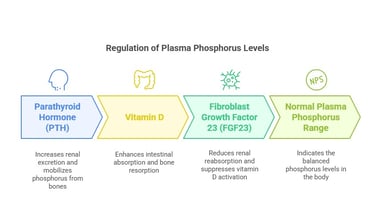
Regulation of Plasma Phosphorus
Plasma phosphorus levels are tightly regulated by:
Parathyroid Hormone (PTH): Increases renal excretion and mobilizes phosphorus from bones.
Vitamin D: Enhances intestinal absorption and bone resorption.
Fibroblast Growth Factor 23 (FGF23): Reduces renal reabsorption and suppresses vitamin D activation. Normal plasma phosphorus levels range from 2.5 to 4.5 mg/dL.
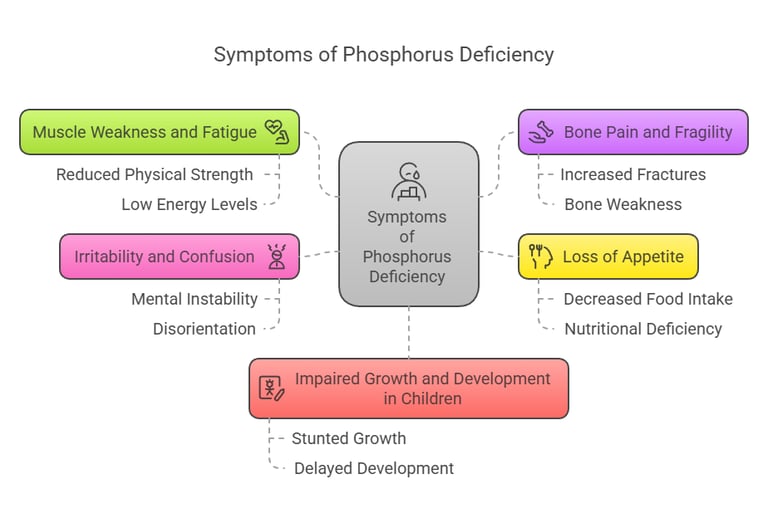
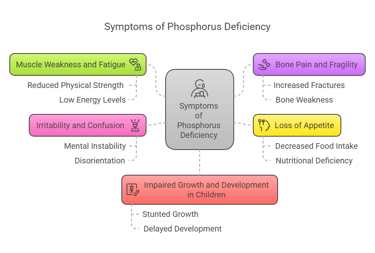
Symptoms of Phosphorus Deficiency
Phosphorus deficiency (hypophosphatemia) is rare but can occur due to malnutrition, alcoholism, or certain medical conditions. Symptoms include:
Muscle weakness and fatigue.
Bone pain and fragility.
Loss of appetite.
Irritability and confusion.
Impaired growth and development in children.
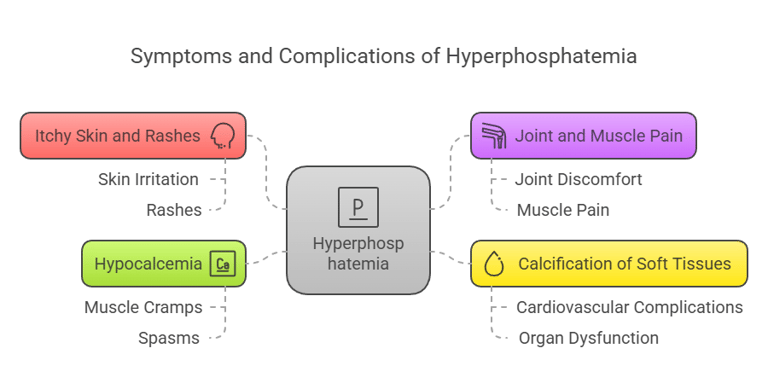
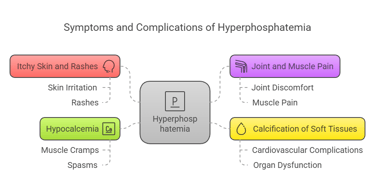
Symptoms of Hyperphosphatemia
Excess phosphorus (hyperphosphatemia) is often linked to kidney dysfunction. Symptoms may include:
Itchy skin and rashes.
Joint and muscle pain.
Calcification of soft tissues, leading to cardiovascular complications.
Hypocalcemia (low calcium levels), causing muscle cramps and spasms.
Magnesium: A Vital Mineral for Health
Body Distribution
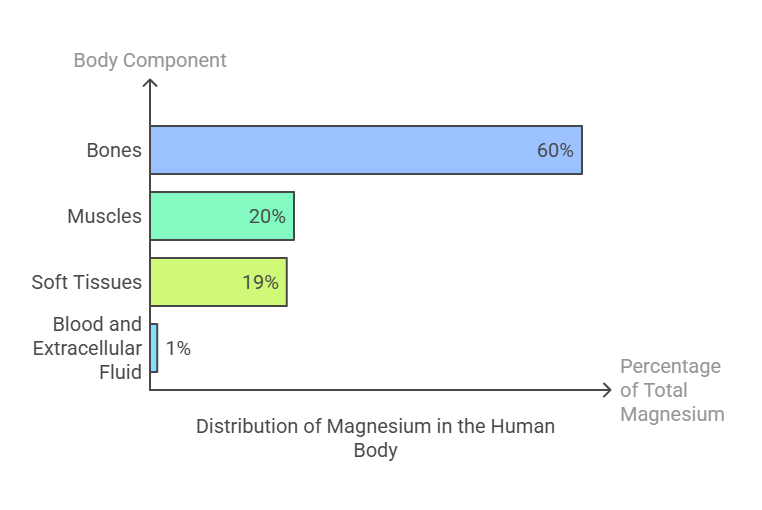
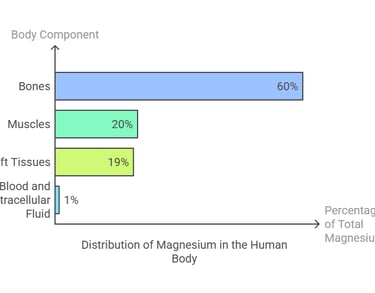
Magnesium is the fourth most abundant mineral in the human body, with a total body content of around 25 grams, distributed as follows:
60% resides in bones, where it contributes to structural strength.
20% is stored in muscles.
19% is found in soft tissues, such as the heart and liver.
1% circulates in the blood and extracellular fluid, playing a crucial role in physiological processes.
Biological Functions
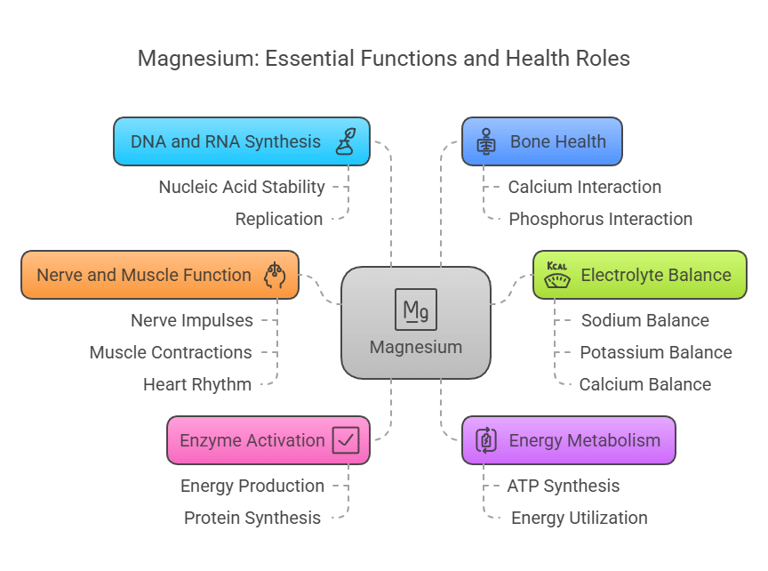
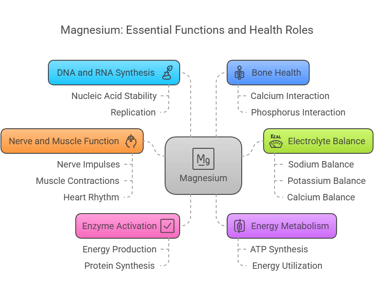
Magnesium is a multitasking mineral involved in numerous essential functions:
Enzyme Activation: Serves as a cofactor for over 300 enzymatic reactions, including those involved in energy production and protein synthesis.
Energy Metabolism: Essential for the synthesis and utilization of ATP (adenosine triphosphate).
DNA and RNA Synthesis: Participates in nucleic acid stability and replication.
Bone Health: Works alongside calcium and phosphorus to maintain bone density.
Nerve and Muscle Function: Regulates nerve impulses, muscle contractions, and heart rhythm.
Electrolyte Balance: Supports proper balance of sodium, potassium, and calcium in cells.
Dietary Requirement
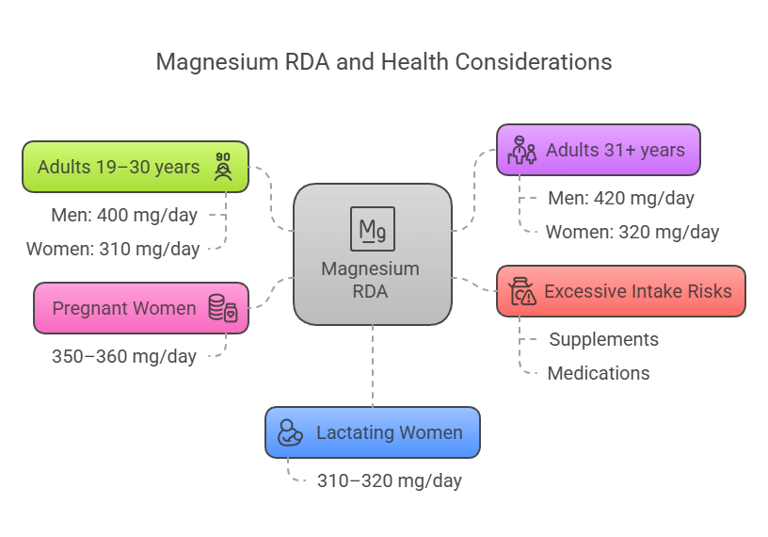
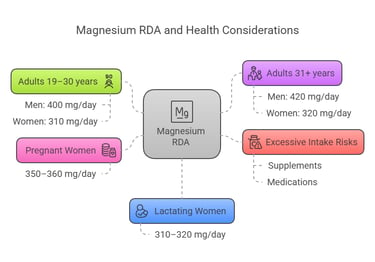
The Recommended Dietary Allowance (RDA) for magnesium depends on age, sex, and physiological conditions:
Adults (19–30 years): Men: 400 mg/day, Women: 310 mg/day.
Adults (31+ years): Men: 420 mg/day, Women: 320 mg/day.
Pregnant women: 350–360 mg/day.
Lactating women: 310–320 mg/day.
Excessive magnesium intake from supplements or medications exceeding 350 mg/day may result in adverse effects like diarrhea.
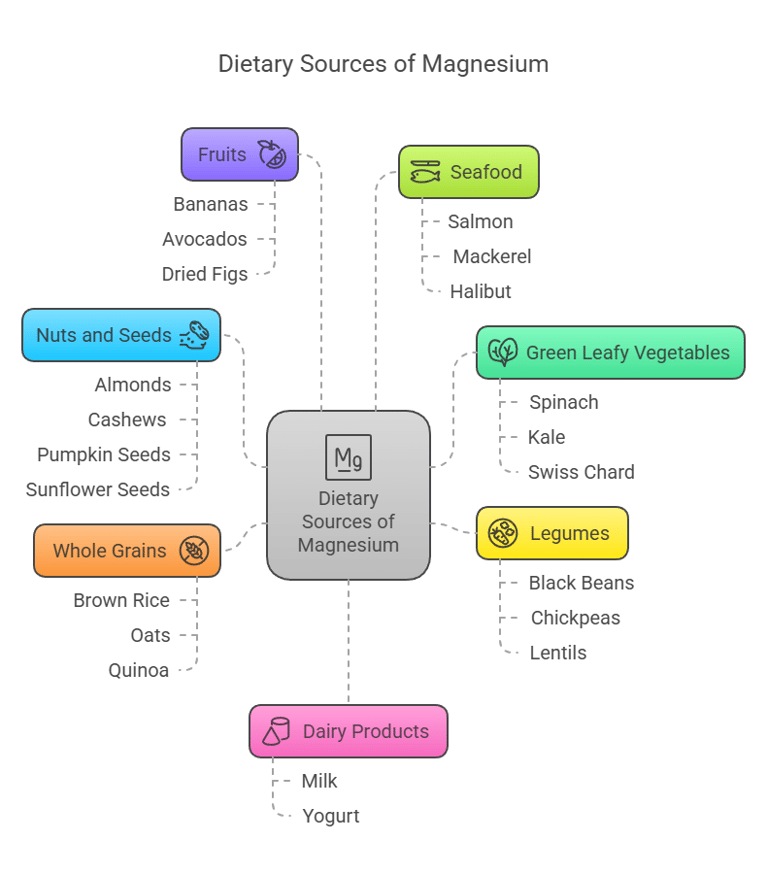
Dietary Sources
Magnesium is found in a wide range of natural foods:
Green Leafy Vegetables: Spinach, kale, and Swiss chard.
Nuts and Seeds: Almonds, cashews, pumpkin seeds, and sunflower seeds.
Legumes: Black beans, chickpeas, and lentils.
Whole Grains: Brown rice, oats, and quinoa.
Fruits: Bananas, avocados, and dried figs.
Seafood: Salmon, mackerel, and halibut.
Dairy Products: Milk and yogurt. Drinking water, especially hard water, may also contribute to magnesium intake.
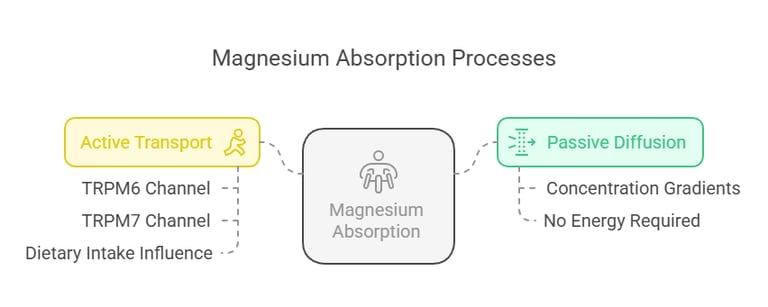
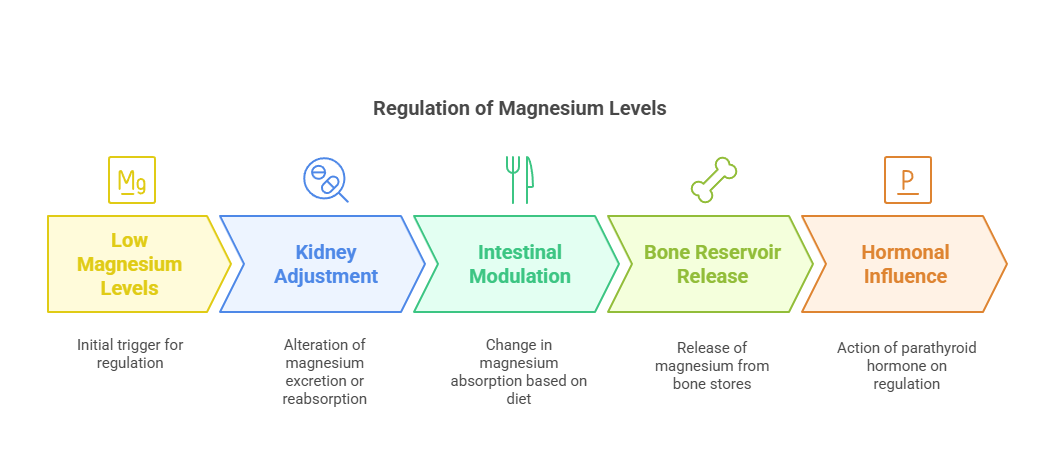
Intestinal Absorption
Magnesium absorption takes place primarily in the small intestine, with 30–50% of dietary magnesium being absorbed. The process involves:
Passive Diffusion: Driven by concentration gradients.
Active Transport: Modulated by magnesium channels such as TRPM6 and TRPM7. Absorption efficiency varies with dietary intake; lower intakes result in higher absorption efficiency.
Symptoms of Magnesium Deficiency
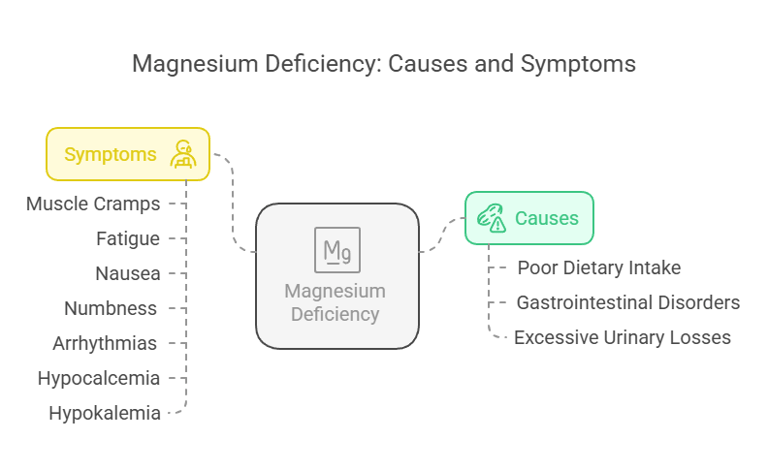
Magnesium deficiency (hypomagnesemia) can occur due to poor dietary intake, gastrointestinal disorders, or excessive losses through urine. Symptoms include:
Muscle cramps, twitches, and spasms.
Fatigue and weakness.
Nausea and vomiting.
Numbness and tingling.
Abnormal heart rhythms (arrhythmias).
Hypocalcemia and hypokalemia (secondary to magnesium deficiency).
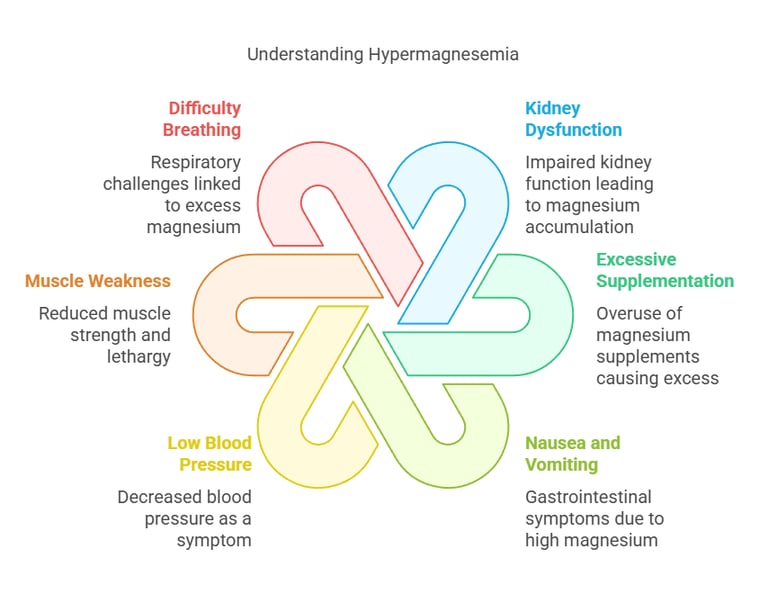
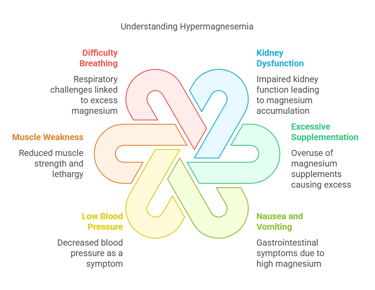
Symptoms of Hypermagnesemia
Excess magnesium (hypermagnesemia) is rare and usually results from kidney dysfunction or excessive magnesium supplementation. Symptoms may include:
Nausea and vomiting.
Low blood pressure (hypotension).
Muscle weakness and lethargy.
Difficulty breathing.
Cardiac arrest in severe cases.
Sodium: A Key Electrolyte in Human Physiology
Body Distribution
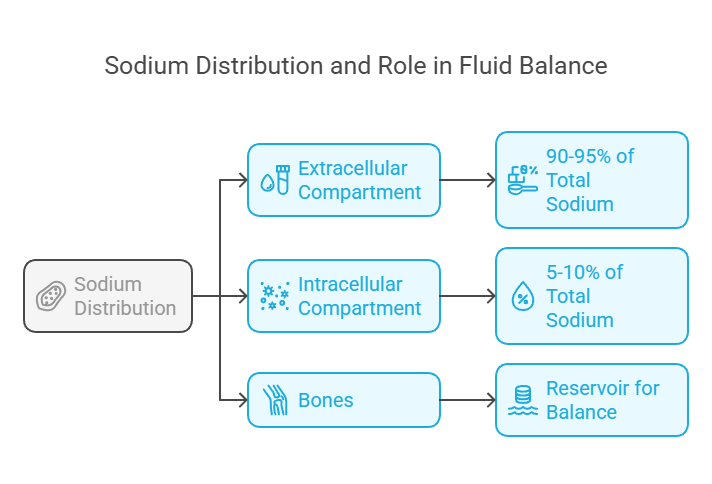
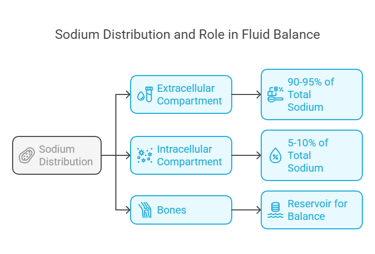
Sodium is primarily found in the extracellular fluid, where it plays a crucial role in maintaining fluid balance. Here's its distribution:
Extracellular Compartment: Around 90–95% of total body sodium resides here, predominantly in blood plasma and interstitial fluid.
Intracellular Compartment: Only a small fraction (5–10%) is inside cells.
Bones: Some sodium is stored in bones as a reservoir for maintaining balance during periods of deficiency.
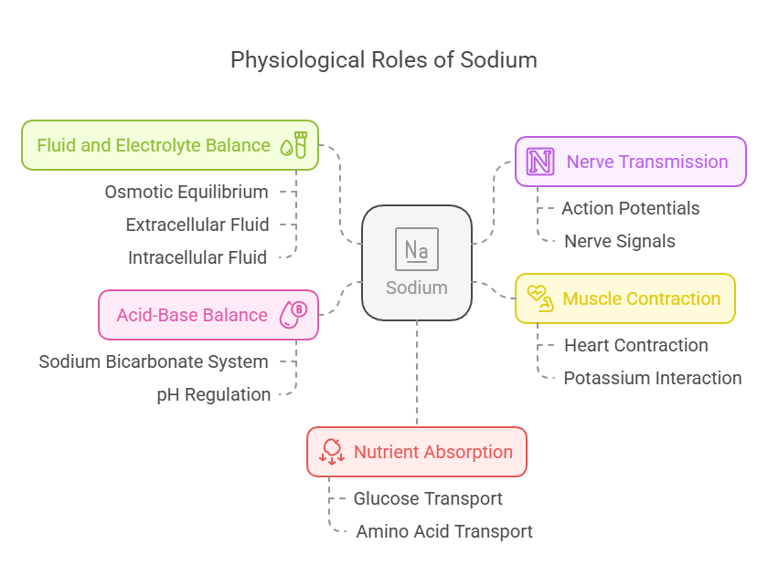
Biological Functions
Sodium is vital for various physiological processes:
Fluid and Electrolyte Balance: Maintains osmotic equilibrium between extracellular and intracellular fluids.
Nerve Transmission: Essential for generating and transmitting action potentials.
Muscle Contraction: Works with potassium to regulate contractions, including those of the heart.
Acid-Base Balance: Contributes to pH regulation via sodium bicarbonate buffer systems.
Nutrient Absorption: Facilitates glucose and amino acid transport in the intestines through sodium-dependent co-transport mechanisms.
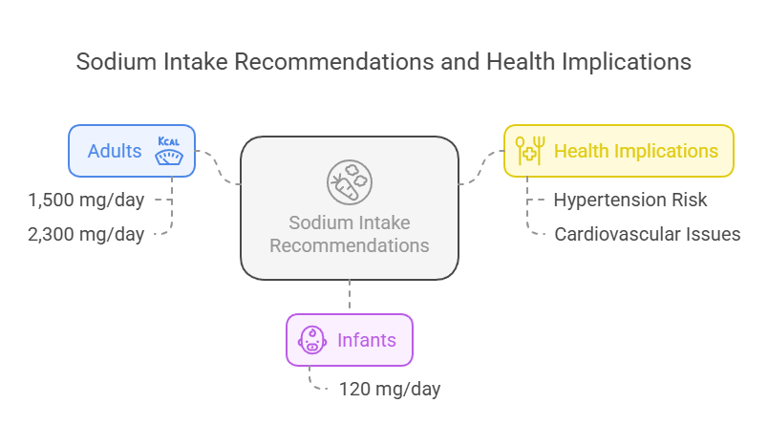
Dietary Requirement
The Recommended Dietary Allowance (RDA) for sodium varies depending on age and health status:
Adults: 1,500 mg/day (Adequate Intake).
Maximum tolerable intake: 2,300 mg/day (to avoid excessive consumption).
Infants (0–6 months): 120 mg/day (based on average breast milk content).
Excessive sodium intake is associated with an increased risk of hypertension and cardiovascular disease, especially in salt-sensitive individuals.
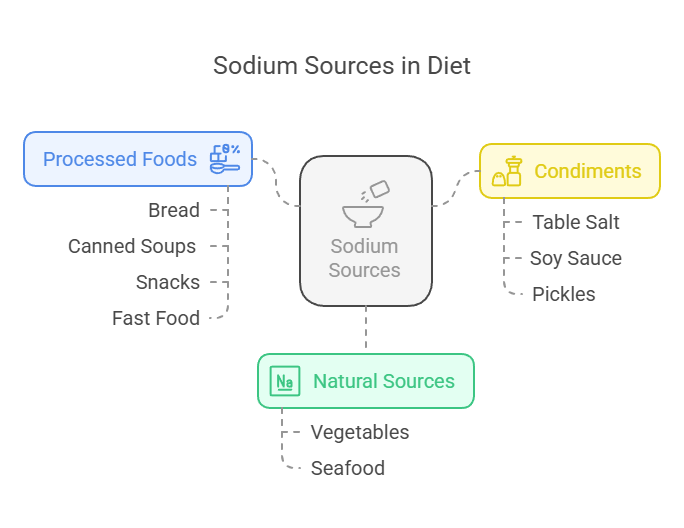
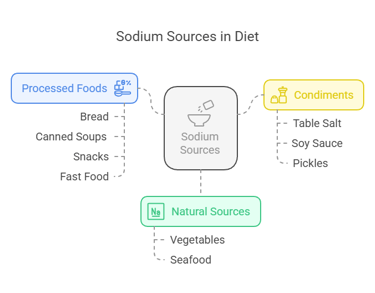
Dietary Sources
Sodium is present in various natural and processed foods:
Natural Sources: Vegetables like celery and beets and seafood such as shrimp and clams.
Processed Foods: Bread, canned soups, snacks like chips, and fast food often have high sodium levels due to added salt.
Condiments: Table salt, soy sauce, and pickles are some common sources of sodium in diets
Clinical Conditions Associated with Hypo and Hypernatremia
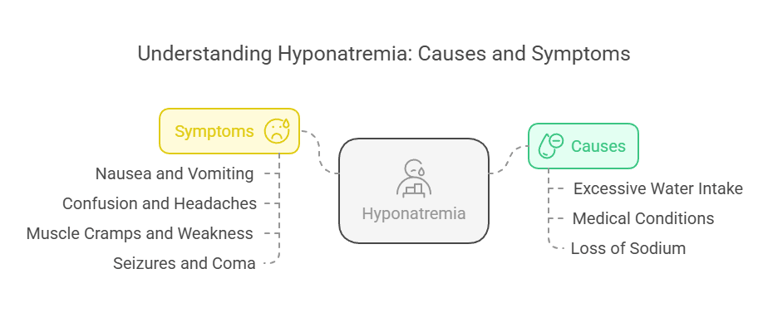
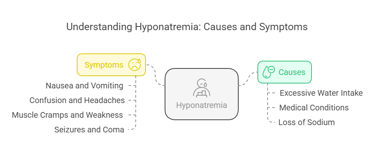
Hyponatremia (low sodium levels) occurs when serum sodium drops below 135 m Eq/L. Causes include:
Excessive Water Intake: Dilutes sodium levels in blood (e.g., polydipsia).
Medical Conditions: SIADH (Syndrome of Inappropriate Antidiuretic Hormone) or heart/kidney failure.
Loss of Sodium: This occurs through diarrhea, vomiting, or excessive sweating.
Symptoms:
Nausea and vomiting.
Confusion and headaches.
Muscle cramps and weakness.
Severe cases can lead to seizures and coma.
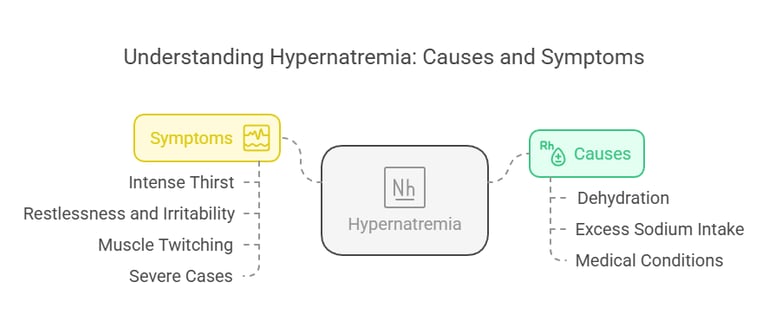
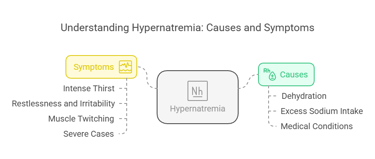
Hypernatremia (high sodium levels) arises when serum sodium exceeds 145 m Eq/L, often due to:
Dehydration: Insufficient water intake or loss of water through fever or sweating.
Excess Sodium Intake: Usually from dietary sources or saline treatments.
Medical Conditions: Diabetes insipidus or impaired thirst mechanism.
Symptoms:
Intense thirst.
Restlessness and irritability.
Muscle twitching.
Severe cases may progress to brain swelling, seizures, and death.
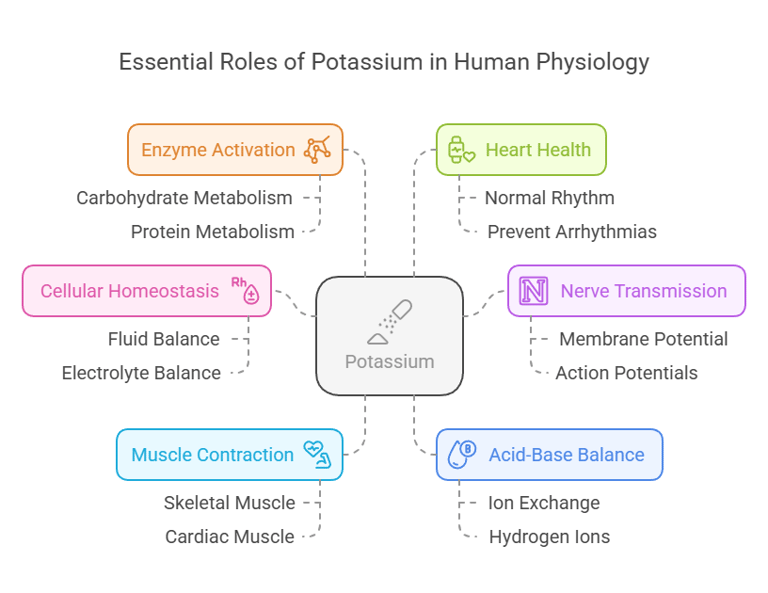
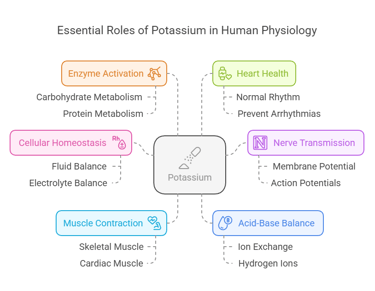
Potassium: A Crucial Electrolyte in Human Health
Biological Functions
Potassium is indispensable for numerous physiological processes:
Cellular Homeostasis: Maintains fluid and electrolyte balance within cells.
Nerve Transmission: Facilitates action potentials in neurons by regulating membrane potential.
Muscle Contraction: Essential for both skeletal muscle movements and cardiac muscle function.
Acid-Base Balance: Helps regulate blood pH by exchanging ions with hydrogen.
Enzyme Activation: Acts as a cofactor for enzymes involved in carbohydrate and protein metabolism.
Heart Health: Plays a vital role in maintaining normal cardiac rhythm and preventing arrhythmias.
Body Distribution
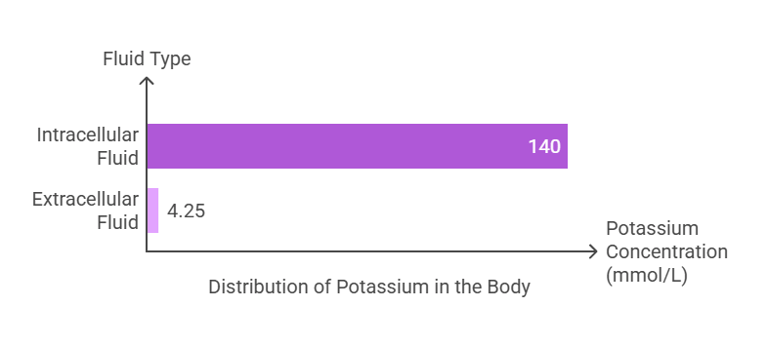
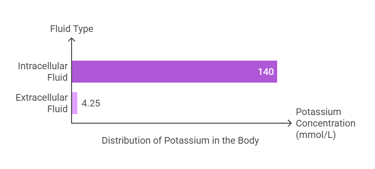
Potassium is the most abundant intracellular cation, with approximately 98% of total body potassium residing within cells:
Intracellular Fluid: The majority of potassium (~140 mmol/L) is found inside cells, where it is critical for cellular functions.
Extracellular Fluid: A small amount (~3.5–5 mmol/L) exists in the bloodstream, playing key roles in neuromuscular and cardiac activities.
The total body potassium content is about 120–160 grams, distributed primarily in muscles and other soft tissues.
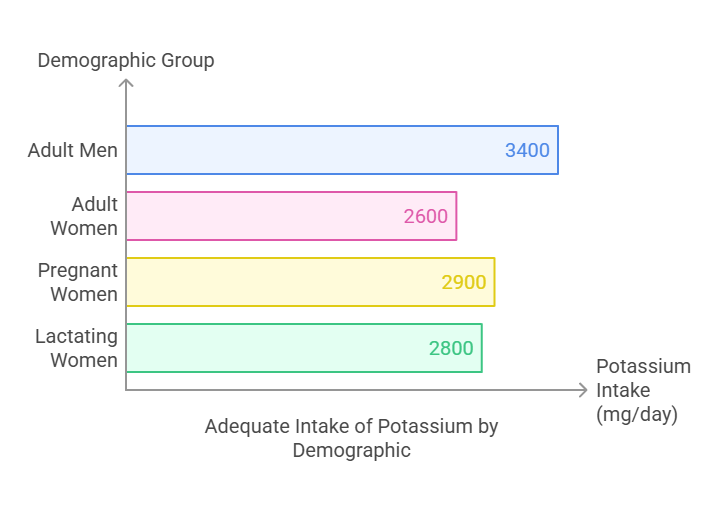
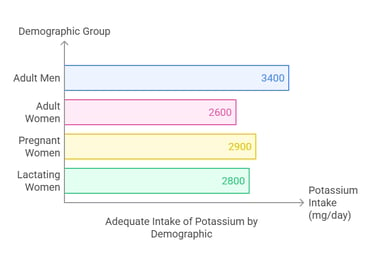
Dietary Requirement
The Adequate Intake (AI) for potassium depends on age and physiological conditions:
Adults (19+ years): 3,400 mg/day for men and 2,600 mg/day for women.
Pregnant women: 2,900 mg/day.
Lactating women: 2,800 mg/day. There is no established upper limit for dietary potassium from natural food sources, as the kidneys efficiently excrete excess amounts. However, excessive supplementation can cause adverse effects.
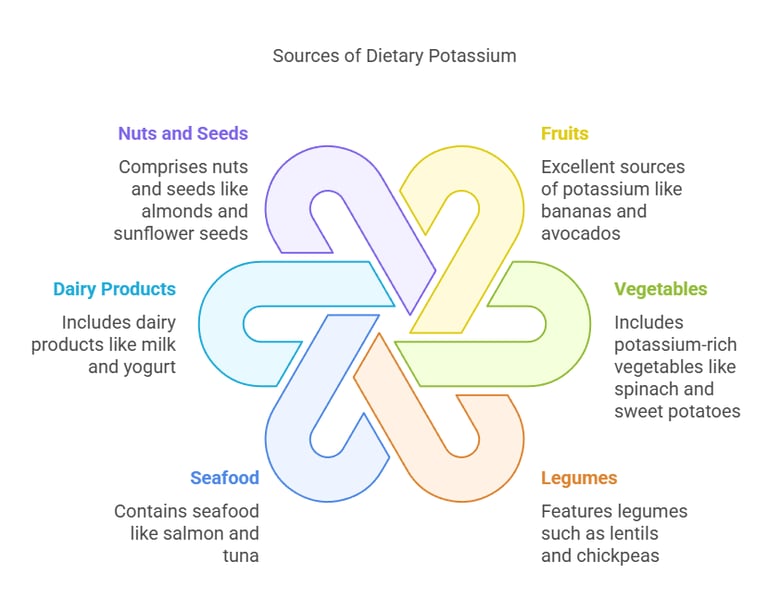
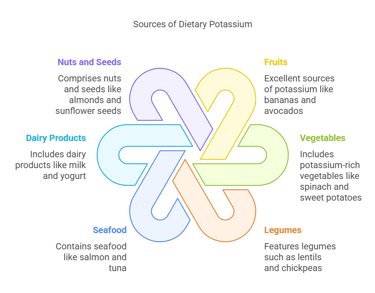
Dietary Sources
Potassium is widely available in both plant-based and animal-based foods:
Fruits: Bananas, oranges, kiwis, and avocados are excellent sources.
Vegetables: Spinach, sweet potatoes, broccoli, and tomatoes.
Legumes: Lentils, chickpeas, and soybeans.
Seafood: Salmon and tuna contain significant amounts of potassium.
Dairy Products: Milk, yogurt, and cheese.
Nuts and Seeds: Almonds, sunflower seeds, and cashews. Potassium-rich diets help lower blood pressure and reduce the risk of stroke and heart disease.
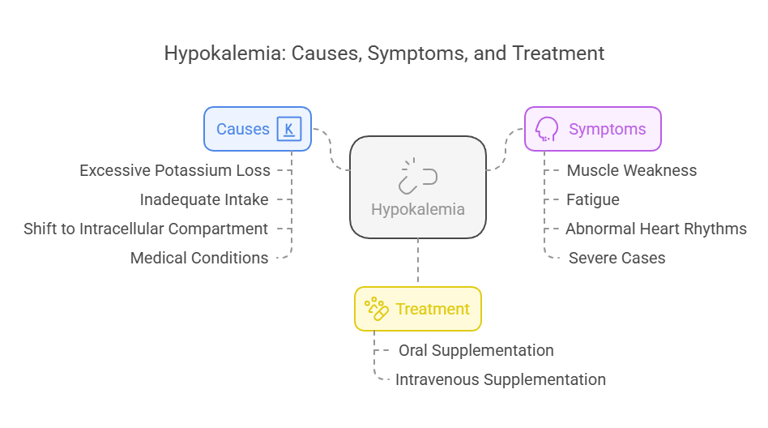
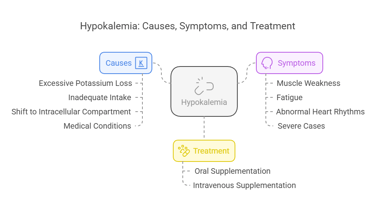
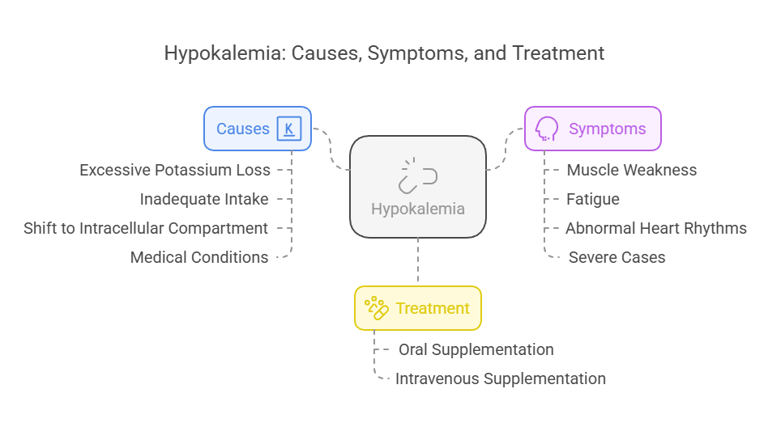
Clinical Conditions Associated with Hypokalemia
Hypokalemia refers to a low serum potassium concentration (<3.5 mmol/L). Causes include:
Excessive Potassium Loss: Through vomiting, diarrhea, or diuretics.
Inadequate Intake: Due to poor diet or malnutrition.
Shift to Intracellular Compartment: Seen in alkalosis or insulin therapy.
Medical Conditions: Hyperaldosteronism or magnesium deficiency.
Symptoms:
Muscle weakness, cramps, and twitching.
Fatigue and lethargy.
Abnormal heart rhythms (e.g., arrhythmias).
Severe cases may result in paralysis or respiratory failure.
Treatment:
Oral or intravenous potassium supplementation, tailored to the severity of the deficiency.
Clinical Conditions Associated with Hyperkalemia
Hyperkalemia occurs when serum potassium levels exceed 5.5 mmol/L, often due to:
Reduced Renal Excretion is common in chronic kidney disease.
Excessive Potassium Intake Through supplements or potassium-rich medications.
Release from Cells: Caused by acidosis, trauma, or hemolysis.
Symptoms:
Muscle weakness and paralysis.
Abnormal heart rhythms, including ventricular fibrillation and cardiac arrest.
Tingling and numbness in extremities.
Treatment:
Medications like calcium gluconate to stabilize heart rhythms.
Insulin and glucose shift potassium into cells.
Dialysis is done in severe cases of kidney dysfunction.
Chlorine (Chloride): An Essential Electrolyte
Body Distribution
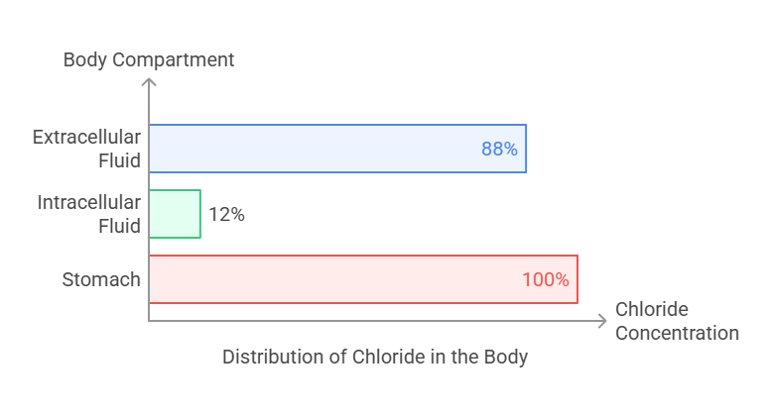
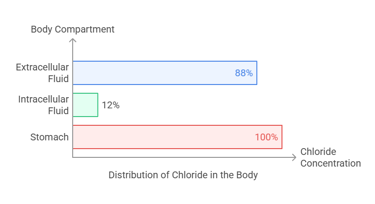
Chloride is predominantly an extracellular anion (negative ion) that complements sodium in maintaining fluid and electrolyte balance. Its distribution includes:
Extracellular Fluid: The majority of chloride (~88%) is found in the plasma and interstitial fluids.
Intracellular Fluid: Around 12% resides inside cells.
Stomach: Concentrated in gastric secretions as hydrochloric acid (HCl), crucial for digestion.
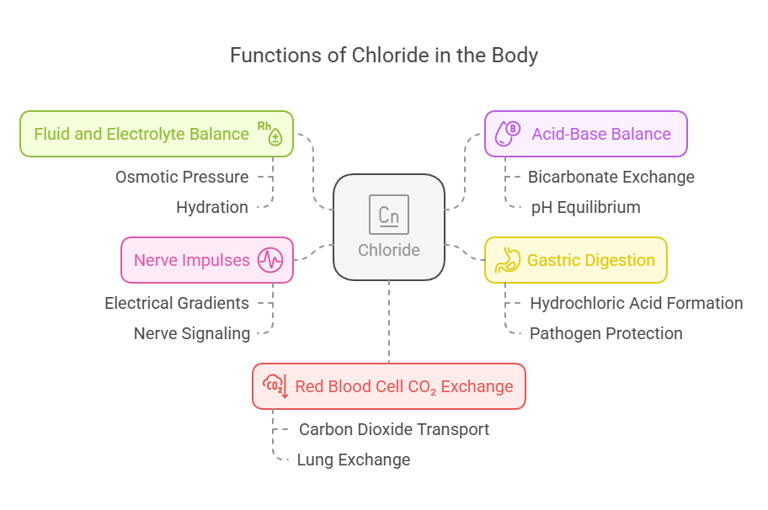
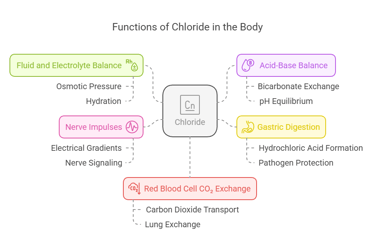
Biological Functions
Chloride serves as a versatile electrolyte with several critical functions:
Fluid and Electrolyte Balance: Works alongside sodium to regulate osmotic pressure and maintain hydration.
Acid-Base Balance: Helps maintain pH equilibrium by participating in bicarbonate exchange processes.
Gastric Digestion: Forms hydrochloric acid (HCl), which aids in breaking down food and protecting against pathogens.
Nerve Impulses: Supports electrical gradients for proper nerve signaling.
Red Blood Cell CO₂ Exchange: Assists in transporting carbon dioxide from tissues to the lungs.
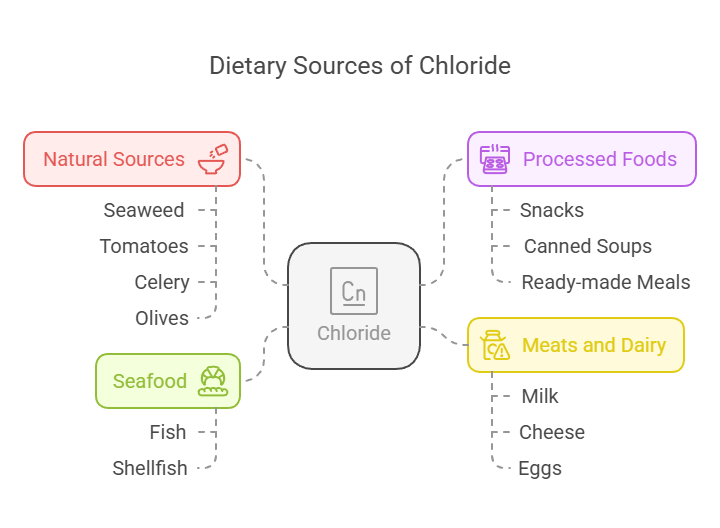
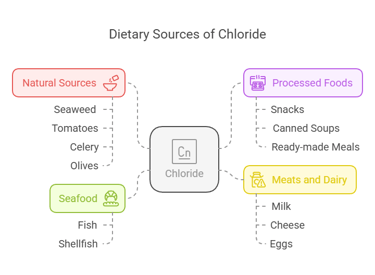
Dietary Sources
Chloride is widely available in food, primarily through its association with sodium in table salt (sodium chloride):
Natural Sources: Seaweed, tomatoes, celery, and olives.
Processed Foods: Snacks, canned soups, and ready-made meals.
Meats and Dairy: Milk, cheese, and eggs.
Seafood: Fish and shellfish are excellent sources.
Dietary Requirement
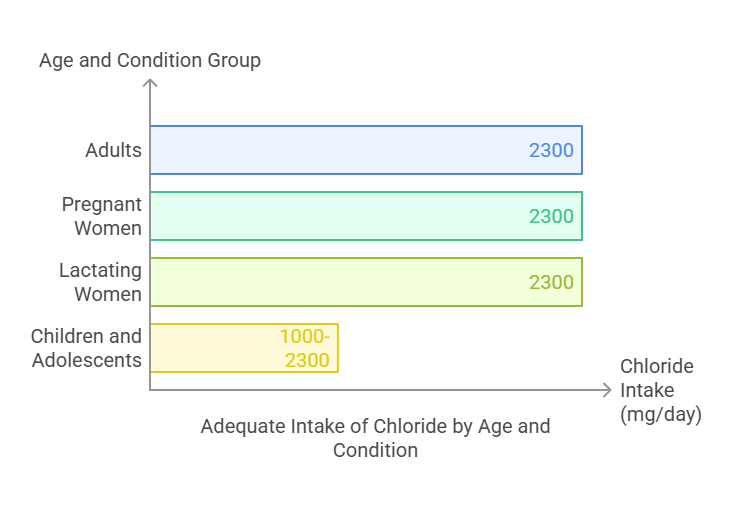
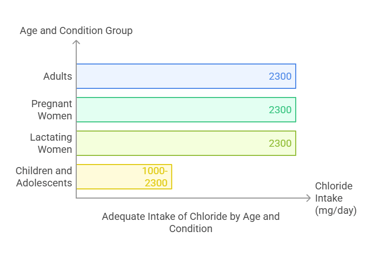
The Adequate Intake (AI) for chloride varies by age and physiological condition:
Adults (19+ years): 2,300 mg/day.
Pregnant women: 2,300 mg/day.
Lactating women: 2,300 mg/day.
Children and adolescents: Ranges from 1,000 to 2,300 mg/day based on age.
Excessive intake of chloride is rare and primarily associated with high salt consumption, which can lead to health concerns such as hypertension.
Clinical Conditions Associated with Hypochloremia
Hypochloremia refers to abnormally low chloride levels (<98 mEq/L) in the blood. Causes include:
Loss of Chloride: Due to vomiting, diarrhea, or excessive sweating.
Medical Conditions: Chronic kidney disease, metabolic alkalosis, or adrenal insufficiency.
Drugs: Diuretics that increase renal chloride excretion.
Symptoms:
Dehydration and muscle weakness.
Fatigue and confusion.
Difficulty breathing (due to impaired acid-base balance).
Treatment:
Addressing the underlying cause and replenishing chloride levels through oral or intravenous supplementation.

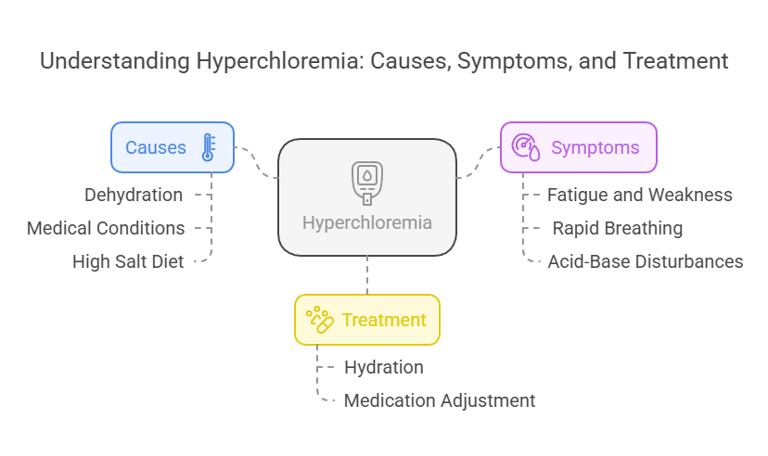
Clinical Conditions Associated with Hyperchloremia
Hyperchloremia refers to elevated chloride levels (>106 m Eq/L) in the blood. Causes include:
Dehydration: Loss of water without proportional chloride loss.
Medical Conditions: Kidney disorders, metabolic acidosis, or excessive saline IV therapy.
High Salt Diet: Leading to excessive chloride intake.
Symptoms:
Fatigue and weakness.
Rapid breathing or hyperventilation.
Acid-base disturbances, causing metabolic acidosis.
Treatment:
Hydration and correction of acid-base balance.
Adjusting medications or saline infusions as needed.
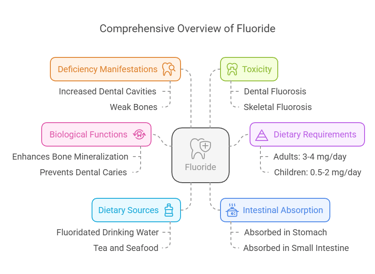
Essential Trace Elements
Body Distribution
Found mainly in bones and teeth, contributing to their structural integrity.
Small amounts exist in soft tissues and plasma.
Biological Functions
Enhances the mineralization of bones and teeth.
Prevents dental caries by reducing enamel demineralization and promoting remineralization.
Dietary Requirements
Adults: 3-4 mg/day.
Children: 0.5-2 mg/day.
Dietary Sources
Drinking water (fluoridated).
Tea, seafood, and fish bones.
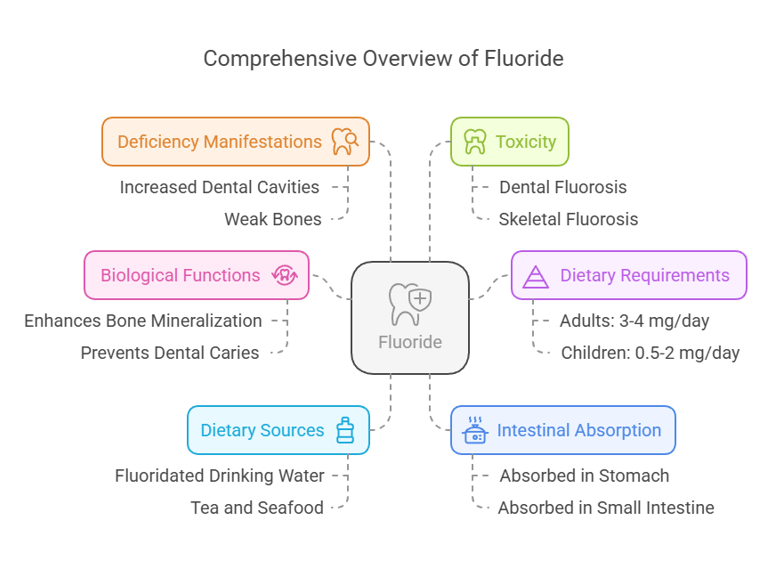
Fluorine (Fluoride)
Intestinal Absorption
Absorbed in the stomach and small intestine.
Approximately 75–90% of ingested fluoride is absorbed.
Deficiency Manifestations
Increased risk of dental cavities.
Weak bones, predisposing to osteoporosis.
Toxicity
Dental fluorosis: Mottling of teeth due to excessive fluoride.
Skeletal fluorosis: Bone pain and stiffness in severe cases.
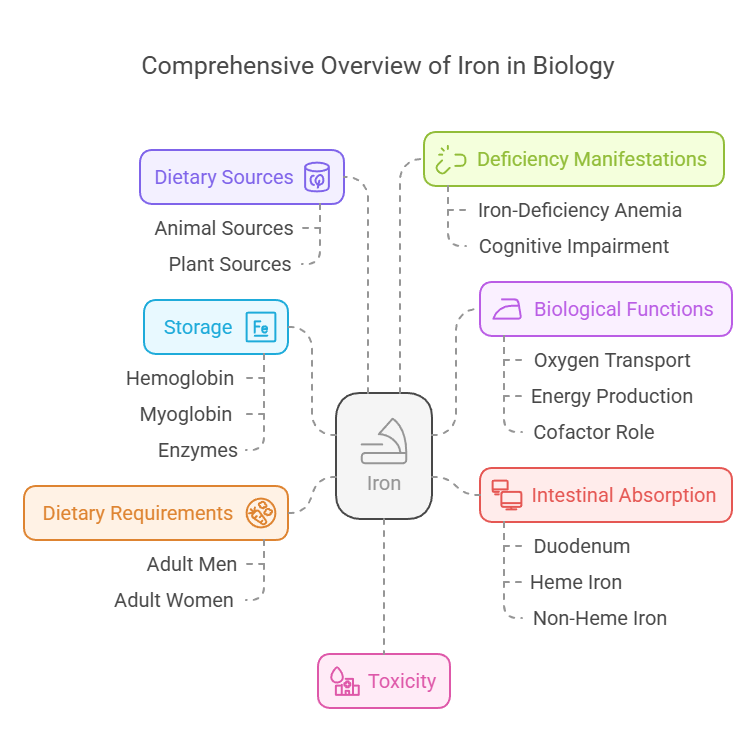
2. Iron
Body Distribution
Stored in hemoglobin (65%), myoglobin, and enzymes.
Found in the liver, spleen, and bone marrow.
Biological Functions
Oxygen transport via hemoglobin.
Energy production in the electron transport chain.
Acts as a cofactor for enzymes.
Dietary Requirements
Adult men: 8 mg/day.
Adult women (19–50 years): 18 mg/day.
Dietary Sources
Animal: Liver, red meat, fish.
Plant: Spinach, legumes, fortified cereals.
Intestinal Absorption
Absorbed in the duodenum.
Heme iron (from animal sources) has higher bioavailability than non-heme iron.
Deficiency Manifestations
Iron-deficiency anemia: Fatigue, pallor, and shortness of breath.
Impaired cognitive development in children.
Toxicity
Iron overload (hemochromatosis): Organ damage due to excess deposition.
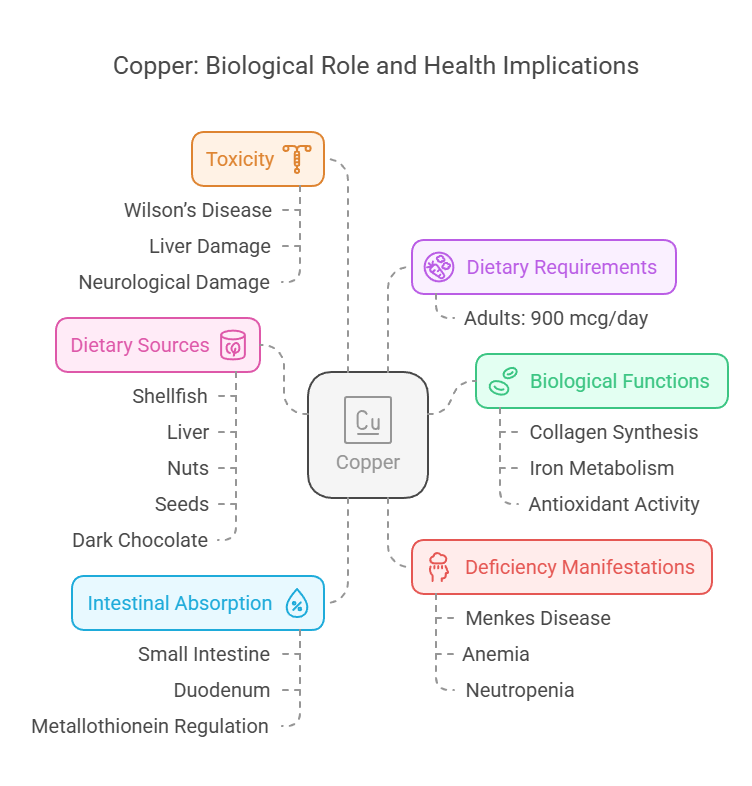
3. Copper
Body Distribution
Found in the liver, brain, heart, and kidneys.
Incorporated into enzymes and plasma proteins like ceruloplasmin.
Biological Functions
Assists in collagen synthesis.
Supports iron metabolism and hemoglobin formation.
Acts as an antioxidant in enzyme systems.
Dietary Requirements
Adults: 900 mcg/day.
Dietary Sources
Shellfish, liver, nuts, seeds, and dark chocolate.
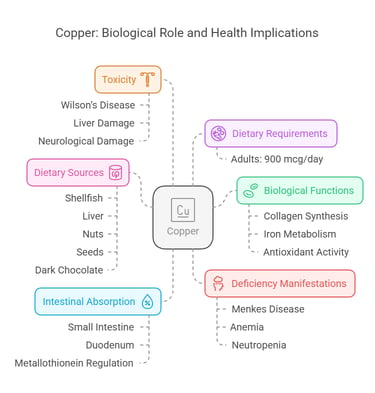
Intestinal Absorption
Absorbed in the small intestine, mostly in the duodenum.
Regulated by proteins like metallothionein.
Deficiency Manifestations
Menkes disease: Brittle hair, growth retardation.
Anemia and neutropenia.
Toxicity
Wilson’s disease: Copper accumulation causing liver and neurological damage.
4. Zinc
Intestinal Absorption
Absorbed in the jejunum and ileum.
Regulated by intestinal zinc-binding proteins.
Deficiency Manifestations
Growth retardation and delayed wound healing.
Impaired immune function and taste alteration.
Toxicity
High doses cause nausea, vomiting, and immune dysfunction.
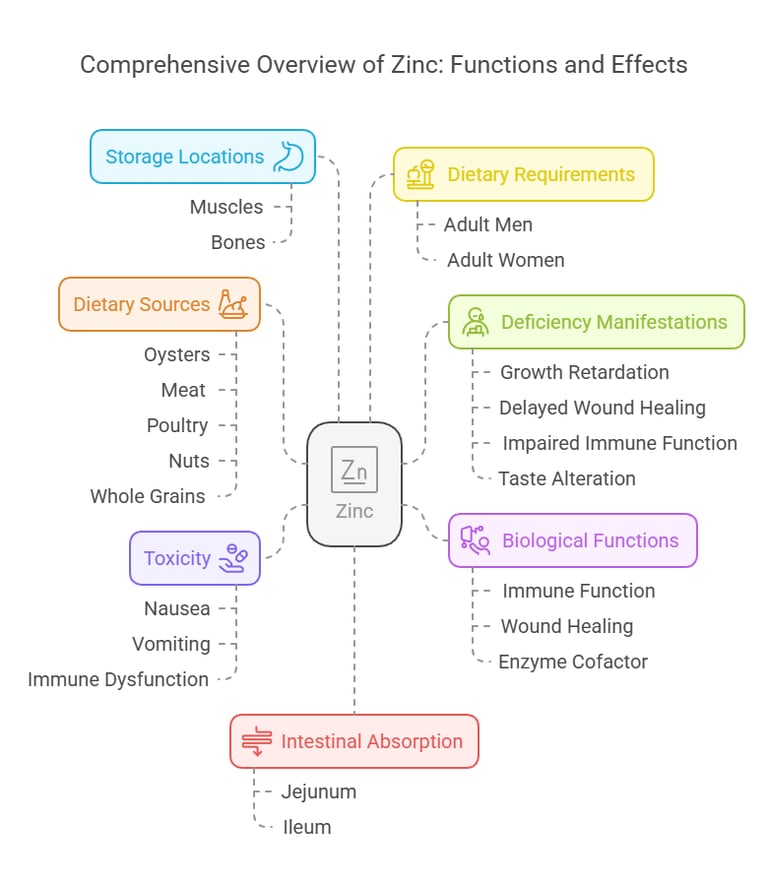
Body Distribution
Predominantly stored in muscles and bones.
Found in the prostate gland, skin, and liver.
Biological Functions
Key role in immune function.
Supports wound healing and cell growth.
It is a cofactor for over 300 enzymes.
Dietary Requirements
Adult men: 11 mg/day.
Adult women: 8 mg/day.
Dietary Sources
Oysters, meat, poultry, nuts, and whole grains.
5. Iodine
Body Distribution
Concentrated in the thyroid gland (>70%).
Found in lesser amounts in tissues like the salivary glands.
Biological Functions
Essential for thyroid hormone synthesis (T3 and T4).
Regulates metabolic processes and growth.
Dietary Requirements
Adults: 150 mcg/day.
Dietary Sources
Iodized salt, seafood, dairy products, and seaweed.
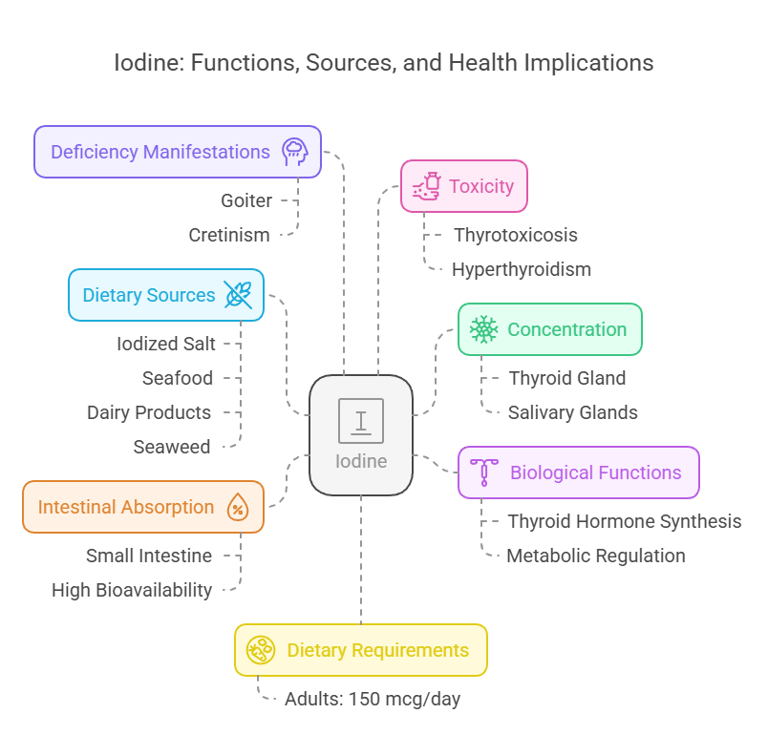
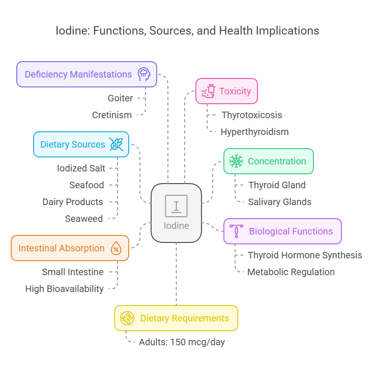
Intestinal Absorption
Rapid absorption in the small intestine.
Nearly 100% bioavailable from dietary sources.
Deficiency Manifestations
Goiter: Thyroid gland enlargement.
Cretinism: Growth and intellectual impairment.
Toxicity
Excess iodine may lead to thyrotoxicosis (hyperthyroidism).
6. Manganese
Intestinal Absorption
Absorbed in the small intestine.
Limited absorption (~3–5%).
Deficiency Manifestations
Bone abnormalities, and impaired glucose tolerance. Growth retardation & fatty infiltration in hepatocytes.
Poor wound healing.
Toxicity
Neurological symptoms resembling Parkinson’s disease (manganese madness).
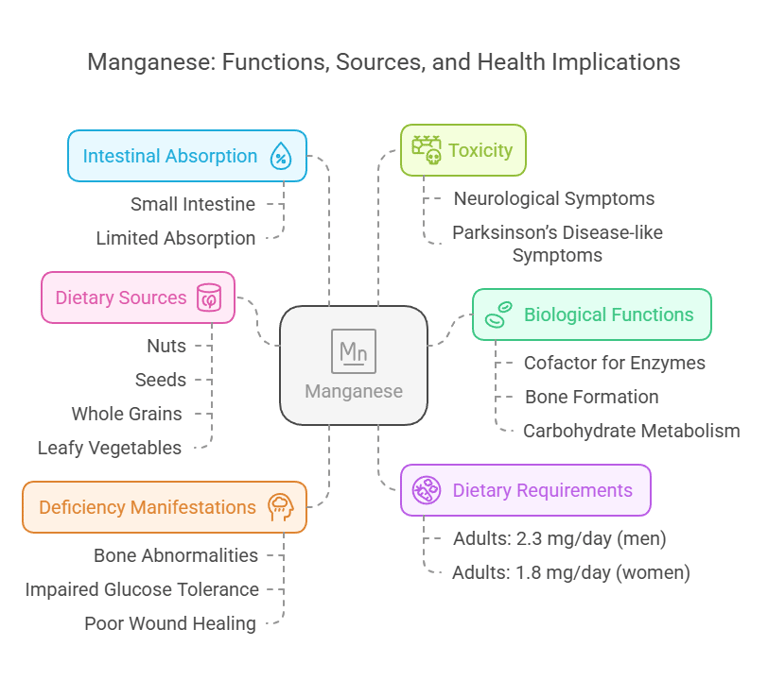
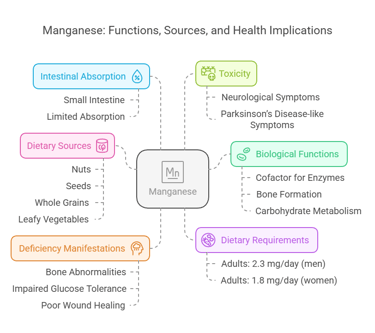
Body Distribution
Stored in bones, liver, pancreas, and kidneys.
Biological Functions
Cofactor for antioxidant enzymes like superoxide dismutase (SOD).
Supports bone formation and carbohydrate metabolism.
Dietary Requirements
Adults: 2.3 mg/day (men), 1.8 mg/day (women).
Dietary Sources
Nuts, seeds, whole grains, and leafy vegetables.
7. Selenium
Body Distribution
Found in muscle tissues, liver, and kidneys.
Incorporated into selenoproteins like glutathione peroxidase.
Biological Functions
Antioxidant defense.
Supports thyroid function and immune health.
Dietary Requirements
Adults: 55 mcg/day.
Dietary Sources
Brazil nuts, fish, eggs, and grains.
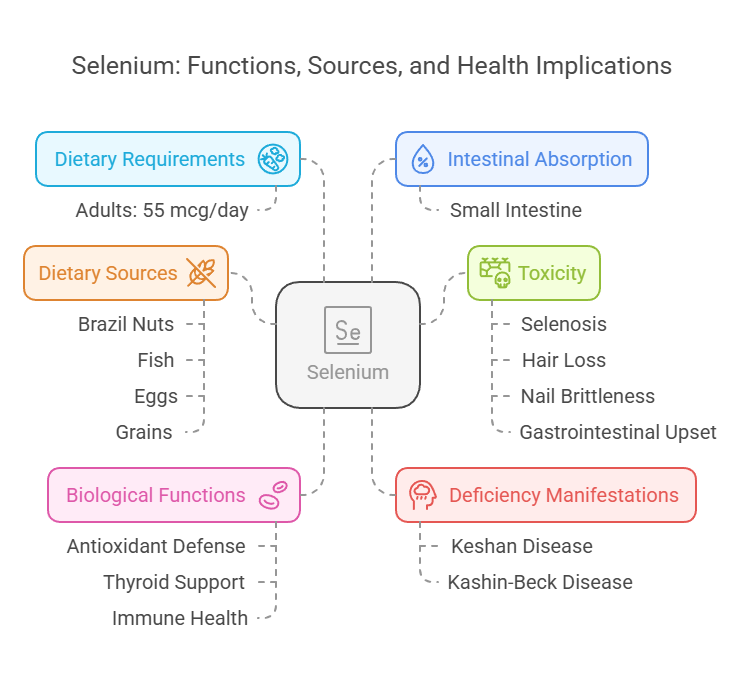
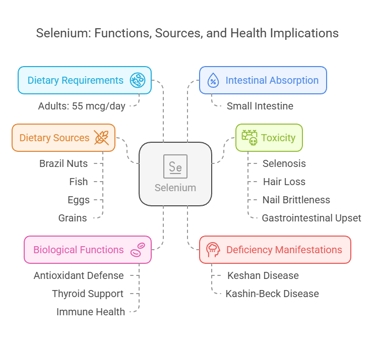
Intestinal Absorption
Absorbed in the small intestine efficiently (>80%).
Deficiency Manifestations
Keshan disease: Cardiomyopathy.
Kashin-Beck disease: Joint degradation.
Toxicity
Selenosis: Hair loss, nail brittleness, and gastrointestinal upset.
8. Chromium
Body Distribution
Stored in small quantities in liver, spleen, and bone.
Biological Functions
Enhances insulin action and glucose metabolism.
Plays a role in lipid metabolism.
Dietary Requirements
Adults: 35 mcg/day (men), 25 mcg/day (women).
Dietary Sources
Broccoli, whole grains, nuts, and meats.
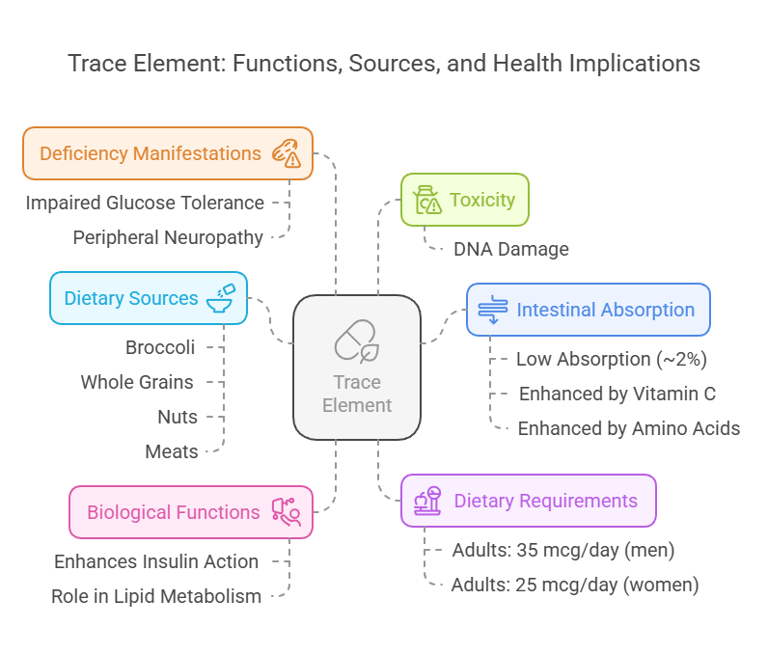
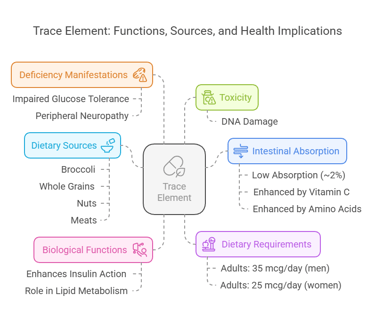
Intestinal Absorption
Absorption is low (~2%).
Enhanced by vitamin C and amino acids.
Deficiency Manifestations
Impaired glucose tolerance.
Peripheral neuropathy.
Toxicity
Rare, but excessive exposure may cause DNA damage.
BLOG
Join us to explore medical biochemistry intricacies.
WRITE TO US
© 2024. All rights reserved.
