Fat-Soluble Vitamins
🟢 Definition
Fat-soluble vitamins are a group of vitamins that dissolve in lipids (fats and oils) rather than water. They are absorbed along with dietary fats in the intestine and are stored in the body's fatty tissues and the liver for later use.
🟢 Introduction
Fat-soluble vitamins (A, D, E, and K) are micronutrients that dissolve in fats and oils, and they are absorbed in the small intestine along with dietary lipids. Unlike water-soluble vitamins, they are stored in the liver and adipose tissue, which means the body has reserves but also a higher risk of toxicity if taken in excess. These vitamins play vital roles in vision, bone health, antioxidant defense, and blood clotting, making them indispensable for survival.
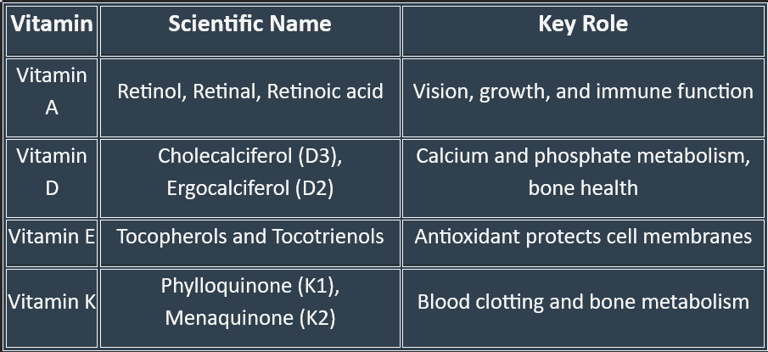
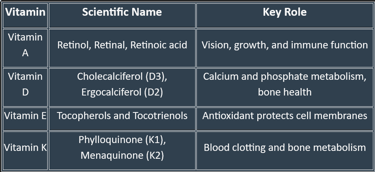
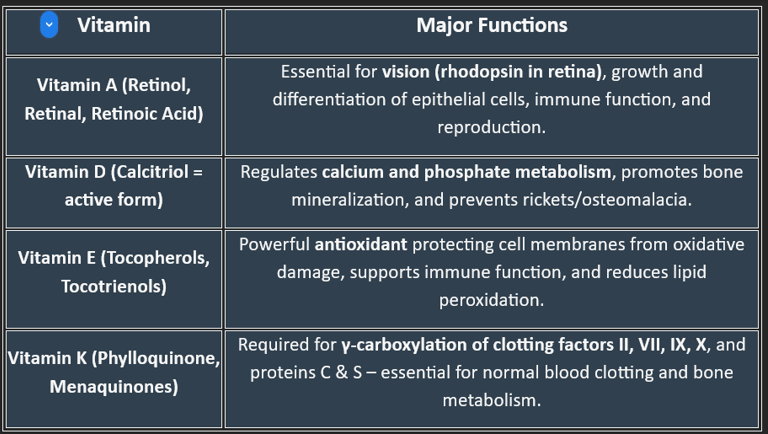
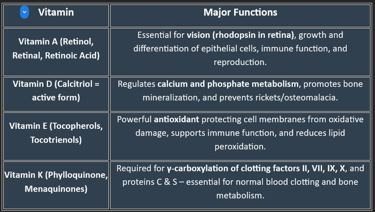
Classification of Fat-Soluble Vitamins
🟢 Based on Chemical Nature & Active Forms
Vitamin A (Retinoids & Carotenoids)
Active forms: Retinol, Retinal, Retinoic acid
Provitamin: β-carotene (from plants)
Vitamin D (Calciferols)
Vitamin D₂ (Ergocalciferol) – from plant sources
Vitamin D₃ (Cholecalciferol) – from animal sources & skin synthesis
Active form in body: Calcitriol (1,25-dihydroxycholecalciferol)
Vitamin E (Tocopherols & Tocotrienols)
Most active form: α-Tocopherol
Vitamin K (Quinones)
K₁ (Phylloquinone) – from plants
K₂ (Menaquinone) – synthesized by intestinal bacteria
K₃ (Menadione) – synthetic, water-soluble form
🟢 Based on Primary Function
Vitamin A → Vision & epithelial health
Vitamin D → Calcium–phosphorus metabolism & bone health
Vitamin E → Antioxidant defense
Vitamin K → Coagulation (blood clotting factors)
✅ Quick Summary:
Fat-soluble vitamins are stored in the body → deficiencies develop slowly, but toxicity risk is higher than with water-soluble vitamins.
Each has a signature role: A for vision, D for bones, E for antioxidant defense, and K for clotting.
Deficiencies lead to characteristic diseases still relevant in clinical practice.
Fat-Soluble Vitamin A
Functions, Requirements, Plasma Levels, Clinical Aspects, Deficiency, Regulation, and Biological Importance
Vitamin A is a fat-soluble vitamin essential for vision, immune health, reproduction, and cellular growth. It exists in two main forms:
Retinoids (Active form) – Found in animal products (retinol, retinal, retinoic acid).
Carotenoids (Precursors) – Found in plants (β-carotene, α-carotene, lycopene).
Vitamin A plays a crucial role in several physiological processes:
Vision – Forms retinal, essential for night vision and color perception.
Immune Function – Enhances immune response by maintaining epithelial barrier integrity.
Cell Growth & Differentiation – Regulates gene transcription, supporting tissue regeneration.
Reproductive Health – Essential for fertility, fetal development, and embryonic growth.
Skin & Mucosal Health – Supports wound healing and maintains healthy skin structure.
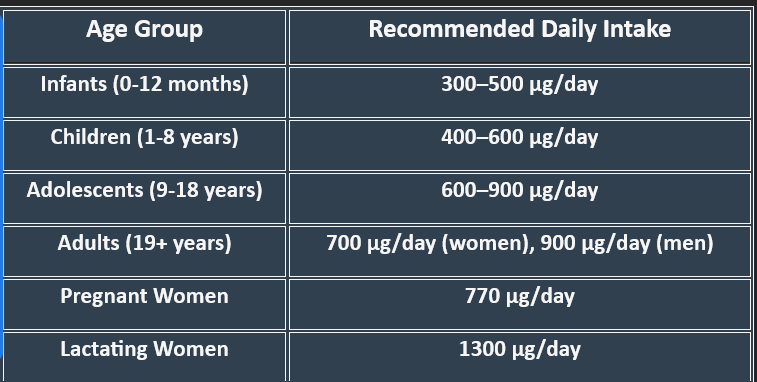
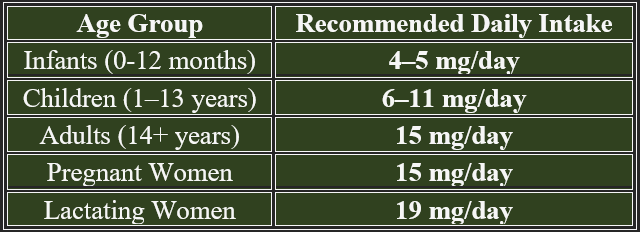
2. Daily Requirements of Vitamin A
1. Functions of Vitamin A
3. Dietary Sources of Vitamin A
Animal-Based Sources – Liver, fish oils, dairy, egg yolks.
Plant-Based Sources – Carrots, sweet potatoes, spinach, mangoes, and tomatoes.
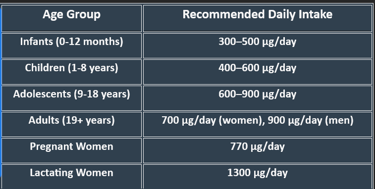
4. Plasma Levels of Vitamin A
5. Clinical Aspects of Vitamin A
Vitamin A levels are measured as retinol concentration in blood.
Low retinol levels increase susceptibility to infections and impaired vision, whereas excessive intake can lead to hypervitaminosis A.
Vitamin A is widely used in medical treatments:
Night Blindness & Xerophthalmia – Prevents vision impairment caused by vitamin A deficiency.
Dermatological Conditions (Acne, Psoriasis) – Used in retinoid therapies for skin renewal.
Measles Treatment – Reduces measles-associated complications in children.
Cancer Prevention – Regulates gene expression to reduce the risk of tumor progression.
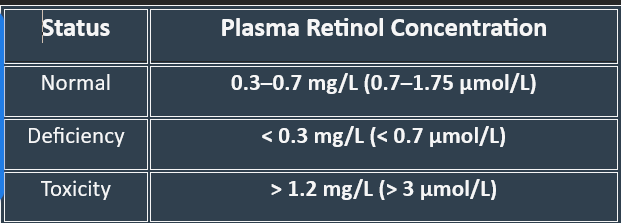

Deficiency leads to serious vision, immune, and growth-related disorders. Common risk factors include malnutrition, malabsorption disorders (such as Crohn’s disease and cystic fibrosis), and liver disease.
6. Vitamin A Deficiency: Manifestations

7. Regulation of Vitamin A
Vitamin A metabolism and storage are tightly regulated:
A. Absorption & Transport
Retinol is absorbed in the small intestine with dietary fats.
Transported via chylomicrons and bound to retinol-binding protein (RBP) for delivery.
B. Storage & Utilization
Stored primarily in the liver, ensuring long-term availability.
Released into circulation as needed to maintain plasma levels.
C. Factors Affecting Regulation
Zinc & Iron Deficiency – Impairs vitamin A metabolism.
Excess Alcohol Consumption – Reduces liver storage and increases deficiency risk.
Vitamin A is critical for survival due to its impact on:
Vision preservation, preventing blindness.
Immune strength reduces infection rates.
Cellular differentiation, ensuring tissue renewal.
Fertility and fetal growth support reproductive health.
8. Biological Importance of Vitamin A
9. Natural Forms of Vitamin A
11. Biosynthesis of Vitamin A
10. Chemistry of Vitamin A
12. Absorption of Vitamin A
13. Transport of Vitamin A
14. Storage of Vitamin A
Natural Forms of Vitamin A
A. Animal-Based Sources (Retinoids)
Liver (richest source)
Dairy products (milk, butter, cheese)
Fish oils (cod liver oil)
Egg yolks
B. Plant-Based Sources (Carotenoids)
Carrots, sweet potatoes, mangoes (β-carotene)
Spinach, kale, pumpkin
Tomatoes, red peppers (Lycopene)
Vitamin A consists of retinoids and carotenoids, each possessing specific chemical structures:
A. Retinoids (Active Forms)
Derived from animal sources, structurally related to retinol.
Common forms include:
Retinol (C₁₀H₁₆O) – Alcohol form, highly active.
Retinal (C₂₀H₂₈O) – Aldehyde form, essential for vision.
Retinoic Acid (C₂₀H₂₈O₂) – Acid form, involved in gene regulation.
B. Carotenoids (Provitamin A)
Found in plant sources, requiring conversion into active vitamin A.
Major carotenoids:
β-Carotene (C₄₀H₅₆) – Most potent precursor.
α-Carotene & Lycopene – Provide antioxidant protection.
A. Conversion of Carotenoids to Retinoids
β-Carotene is cleaved in the intestine by β-carotene dioxygenase, forming retinal.
Retinal is converted to retinol and stored in the liver.
Retinol is oxidized to retinoic acid, which regulates gene expression.
B. Storage & Metabolism
Stored in hepatic stellate cells as retinyl esters.
Released as needed and transported via retinol-binding protein (RBP).
Excess vitamin A is excreted via bile.
Once absorbed, vitamin A follows a specific transport mechanism to reach target tissues:
A. Chylomicron Transport (Initial Phase)
Newly absorbed retinol esters are packaged into chylomicrons for delivery.
Transported via lymphatic circulation to the liver.
B. Liver Processing & Release
Stored as retinyl esters in hepatic stellate cells.
When needed, retinol is bound to retinol-binding protein (RBP) for transport in circulation.
C. Target Tissue Delivery
Retinol enters cells by binding to specific receptors.
In eye tissues, retinol is converted into retinal for vision.
In immune cells, retinoic acid modulates gene expression for immune function.
Factors Enhancing Absorption:
✔ Presence of dietary fats improves absorption.
✔ Bile acids aid in emulsification.
✔ Low-fiber diets prevent interference with absorption.
Factors Reducing Absorption:
❌ Fat malabsorption disorders (e.g., cystic fibrosis, Crohn’s disease).
❌ High fiber or phytate-rich diets, binding retinoids.
❌ Alcohol abuse, impaired intestinal function.
Vitamin A is absorbed in the small intestine, primarily in the duodenum and jejunum. The process differs based on the form of vitamin A:
A. Retinoids (Preformed Vitamin A from Animal Sources)
Absorbed directly in enterocytes after emulsification with bile salts.
Incorporated into chylomicrons for transport via the lymphatic system.
B. Carotenoids (Provitamin A from Plant Sources)
Converted into retinal within intestinal cells by β-carotene dioxygenase.
Further reduced to retinol, then transported similarly to retinoids.
Vitamin A is primarily stored in the liver, ensuring long-term availability:
Stored in hepatic stellate cells as retinyl esters.
Released gradually based on physiological demand.
Excess vitamin A is stored in adipose tissue, preventing toxicity.
Regulation of Storage & Mobilization
✔ Low vitamin A levels → Release of retinol from storage.
❌ Excess vitamin A → Risk of toxicity & liver damage.
Vitamin A and Its Functions in Target Tissues
Vitamin A plays a crucial role in multiple target tissues, ensuring vision, immune defense, reproduction, growth, and cellular differentiation. Its biologically active forms—retinol, retinal, and retinoic acid—execute tissue-specific functions.
Vitamin A is indispensable for tissue-specific functions, ensuring proper vision, immune protection, growth, reproduction, and skin health. Its active forms—retinal, retinol, and retinoic acid—regulate biological processes by modulating gene expression and cellular differentiation.
1. Vision: Retinal in the Retina
2. Immune System: Retinoic Acid in Epithelial & Immune Cells
3. Growth & Development: Retinoic Acid in Embryonic & Bone Tissue
4. Reproductive Health: Retinol in Gonadal Tissue
5. Skin & Epithelial Health: Retinoic Acid in Epidermis
1. Vision: Retinal in the Retina
Target Tissue: Retina (Rod and Cone Cells)
Function:
Retinal binds to opsin proteins to form rhodopsin, the light-sensitive pigment in rod cells.
Enables night vision and adaptation to dim light.
Supports color perception in cone cells.
Deficiency Impact: Night blindness (impaired low-light vision).
2. Immune System: Retinoic Acid in Epithelial & Immune Cells
Target Tissue: Skin, Mucosal Barriers (Respiratory, Gastrointestinal, Urogenital)
Function:
Maintains epithelial integrity, preventing microbial invasion.
Enhances immune responses by regulating T-cell and macrophage function.
Deficiency Impact: Increased infections, respiratory illnesses, gastrointestinal infections.
3. Growth & Development: Retinoic Acid in Embryonic & Bone Tissue
Target Tissue: Fetal tissues, bone cells (osteoblasts, osteoclasts)
Function:
Regulates gene transcription via nuclear receptors (RAR and RXR).
Promotes bone development and epiphyseal growth plate maintenance.
Supports fetal organogenesis (heart, lungs, limbs).
Deficiency Impact: Stunted growth, developmental abnormalities, increased fracture risk.
4. Reproductive Health: Retinol in Gonadal Tissue
Target Tissue: Ovaries, Testes (Germ Cells)
Function:
Essential for spermatogenesis (sperm development) in males.
Supports oocyte maturation and implantation in females.
Deficiency Impact: Infertility, impaired fetal development.
5. Skin & Epithelial Health: Retinoic Acid in Epidermis
Target Tissue: Skin, Hair Follicles
Function:
Enhances keratinocyte differentiation, preventing dryness.
Regulates collagen synthesis, improving skin texture and healing.
Deficiency Impact: Hyperkeratosis (rough, dry skin), increased risk of acne, and premature aging.
Wald’s Cycle: Phototransduction in the Retina
Vitamin A’s Role in Vision
Vitamin A is essential for vision, particularly in low-light conditions. It plays a crucial role in the formation of rhodopsin, a light-sensitive pigment in the retina, and supports color vision and eye health.
Vitamin A is critical for vision, supporting night vision, color perception, and eye tissue health. Deficiency leads to night blindness, dry eye disorders, and potential blindness, emphasizing the importance of adequate intake.
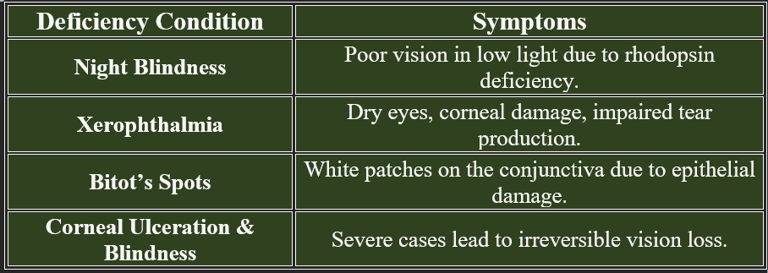
2. Deficiency Manifestations in Vision
1. Function in Vision
A. Formation of Rhodopsin in Rod Cells
Retinal, a form of vitamin A, combines with opsin to form rhodopsin in rod cells.
Rhodopsin absorbs light, triggering a signal transduction pathway that allows vision in dim light.
Low vitamin A levels impair rhodopsin regeneration, leading to night blindness.
B. Role in Cone Cells (Color Vision)
Cone cells in the retina utilize vitamin A for photopsins, allowing color perception.
Deficiency affects color discrimination, reducing visual clarity.
C. Maintenance of Corneal and Conjunctival Health
Retinoic acid maintains epithelial integrity in the cornea and conjunctiva, preventing dry eye and infections.
Deficiency can cause xerophthalmia (dry eyes) and even corneal ulceration, leading to blindness.
3. Regulation of Vitamin A in Eye Function
Dietary intake replenishes retinal stores, maintaining rhodopsin function.
Zinc supports vitamin A metabolism, ensuring efficient conversion into retinal.
Light exposure triggers rhodopsin recycling, preserving night vision.

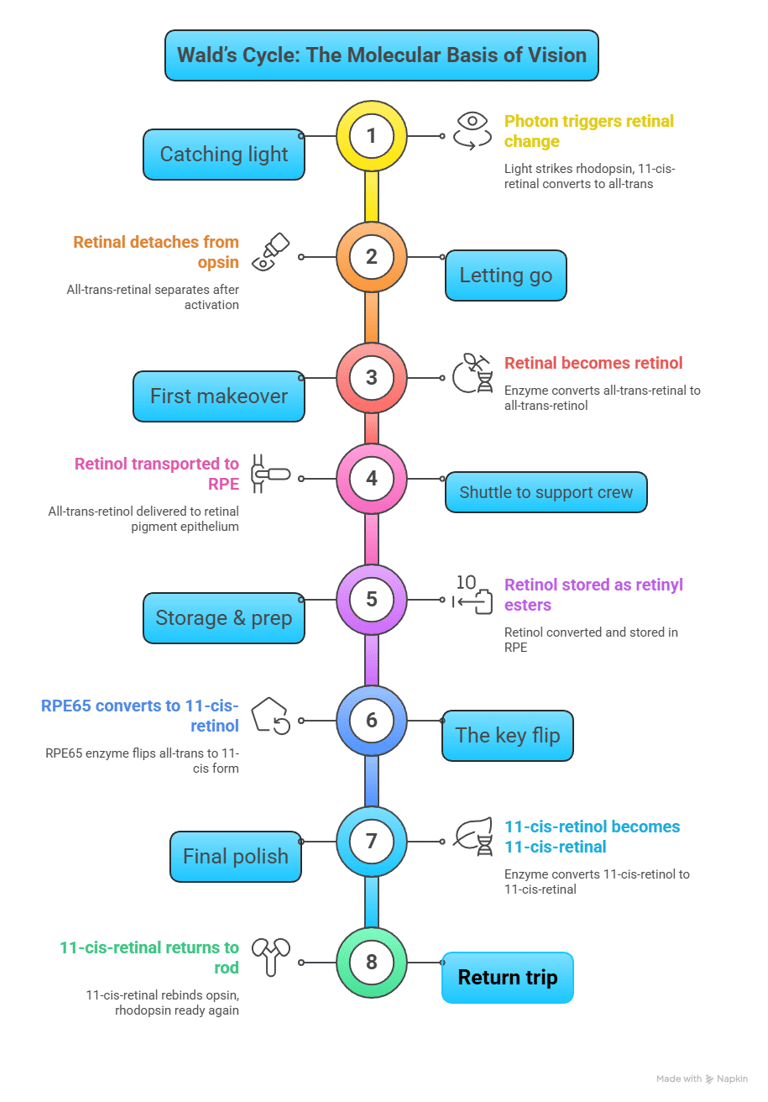
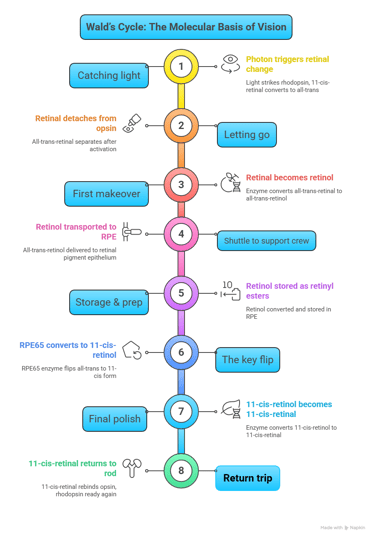
Wald’s Cycle, also known as the visual cycle, describes the biochemical process by which retinal photoreceptors (rods and cones) regenerate light-sensitive pigments, ensuring continuous vision. This cycle was elucidated by George Wald, earning him the Nobel Prize in Physiology or Medicine in 1967.
Imagine your eyes as cameras. For a camera to take one picture after another, the film has to be reset each time. In the eye, the “film” is a special molecule called 11-cis-retinal. Every time light hits it, it changes shape (to all-trans-retinal).
But unless it’s flipped back to its original form, the eye can’t capture the next “photo.”
That resetting process is what Wald’s Cycle is all about.
Step-by-step, in plain words
Catching light (in rods)
In the rod cells of the retina, 11-cis-retinal sits snugly inside a protein called opsin (together they make rhodopsin).
A photon of light strikes, and 11-cis-retinal instantly changes into all-trans-retinal.
This twist switches on rhodopsin and starts the signal that eventually reaches your brain as vision.
Letting go
Once it has done its job, all-trans-retinal detaches from opsin.
First makeover (inside rods)
An enzyme quickly converts all-trans-retinal into all-trans-retinol (a vitamin A alcohol).
Shuttle to the support crew
All-trans-retinol is ferried out of the rod cell to the retinal pigment epithelium (RPE) with the help of transport proteins. Think of this as a delivery truck service.
Storage & prep (in RPE)
Inside the RPE, the molecule is tucked away as retinyl esters for safekeeping.
The key flip (RPE)
Now comes the magic step: an enzyme called RPE65 flips the all-trans form back into 11-cis-retinol.
This is the heart of Wald’s cycle.
Final polish
Another enzyme changes 11-cis-retinol into 11-cis-retinal — the exact shape needed for vision.
Return trip
11-cis-retinal is shipped back to the rod cell, slips into opsin, and rhodopsin is ready again for the next flash of light.
👉Why this cycle matters
Without this recycling, rods and cones would stop working after the first flash of light.
Vitamin A deficiency means there isn’t enough raw material → night blindness.
Faults in the cycle (like RPE65 mutations) can cause severe retinal diseases.
This cycle is a perfect example of teamwork between the photoreceptor cells and the RPE. One catches the light, the other resets the film. Together, they keep our eyes “reloading” so we can see continuously.
👉 Bleach → Release → Reduce → Esterify → Flip (RPE65) → Polish → Return
1. Overview of Wald’s Cycle
Converts 11-cis-retinal (active form) into all-trans-retinal upon light exposure.
Regenerates 11-cis-retinal to maintain photoreceptor function.
Essential for night vision (rod cells) and color perception (cone cells).
2. Steps of Wald’s Cycle (Visual Cycle)
Step 1: Light Activation of Rhodopsin
Retinal photoreceptors (rods) contain rhodopsin, a pigment critical for dim light vision.
Absorption of photons triggers the isomerization of 11-cis-retinal into all-trans-retinal.
Rhodopsin undergoes conformational change, initiating phototransduction, where electrical signals are sent to the brain.
Step 2: Release of All-Trans-Retinal
After activation, rhodopsin dissociates, releasing all-trans-retinal.
All-trans-retinal is transported out of the photoreceptor cells.
Step 3: Conversion to Retinol in Retinal Pigment Epithelium (RPE)
All-trans-retinal is reduced to all-trans-retinol (vitamin A alcohol) by retinal enzymes.
Retinol is transported to the retinal pigment epithelium (RPE), where regeneration occurs.
Step 4: Regeneration of 11-Cis-Retinal
Retinol is esterified to retinyl esters before conversion back to 11-cis-retinal.
Enzymes in RPE facilitate the conversion, restoring the light-sensitive form.
Step 5: Reassembly with Opsin
11-cis-retinal recombines with opsin, forming regenerated rhodopsin, ready for another cycle of light absorption.
3. Biological Importance of Wald’s Cycle
Maintains continuous vision by ensuring photoreceptor sensitivity is restored.
Prevents night blindness, a condition caused by impaired retinal regeneration.
Supports efficient light perception, crucial for low-light and color vision.
Protects photoreceptors from damage, ensuring longevity of retinal function.
Vitamin A plays a crucial role in vision, immunity, cellular differentiation, and reproductive health. Its therapeutic applications extend to dermatology, ophthalmology, oncology, and pediatrics. However, excessive intake can lead to severe toxicity.
1. Clinical Applications of Vitamin A
2. Vitamin A Toxicity (Hypervitaminosis A)
Clinical Applications & Toxicity Risks of Vitamin A
A. Ophthalmology: Treatment of Night Blindness & Xerophthalmia
Vitamin A deficiency leads to impaired vision, particularly in low-light conditions (night blindness).
Used in high-dose supplementation to restore retinal function.
Xerophthalmia (dry eye disease) caused by prolonged deficiency is treated with oral retinol therapy.
B. Dermatology: Acne & Psoriasis Treatment
Retinoids (synthetic vitamin A derivatives) are used in skin conditions like acne and psoriasis.
Isotretinoin (Accutane) is a potent retinoid prescribed for severe acne.
Topical tretinoin enhances skin cell renewal and collagen synthesis.
C. Immunology: Measles Treatment & Immune Enhancement
Measles reduces vitamin A levels, leading to increased disease severity.
WHO recommends vitamin A supplementation for children with measles to lower mortality rates.
Supports immune function by strengthening epithelial barriers.
D. Oncology: Cancer Prevention & Chemoprevention
Vitamin A regulates gene expression, influencing tumor suppression.
Retinoids are studied in preventing and treating leukemia, lung cancer, and skin cancer.
Excess vitamin A may promote tumor growth, requiring careful dosage.
E. Growth & Development: Pediatric Nutrition & Pregnancy Support
Essential for fetal development, preventing congenital defects.
Supports normal growth in children, preventing stunted development.
Excess intake in pregnancy may cause teratogenic effects (birth defects).
A. Acute Toxicity (High single-dose intake)
Symptoms:
Nausea, vomiting, dizziness, blurred vision, headache.
Increased intracranial pressure leading to papilledema (optic nerve swelling).
B. Chronic Toxicity (Prolonged high intake)
Symptoms:
Hypercalcemia, leading to kidney dysfunction.
Bone pain & fractures due to excessive osteoclast activity.
Liver damage from accumulation.
Neurological issues (irritability, headache, cognitive impairment).
C. Teratogenic Effects in Pregnancy
High vitamin A intake (>10,000 IU/day) during pregnancy can cause congenital malformations.
Avoid excessive retinoid use (e.g., isotretinoin in acne treatment).
🌞 Vitamin D: The Sunshine Hormone
📉 Deficiency
Causes: inadequate sunlight, malabsorption, chronic kidney/liver disease
Consequences:
Rickets (children): defective mineralization, bowed legs, rachitic rosary
Osteomalacia (adults): bone pain, fractures, pseudo fractures
Hypocalcemic tetany (if severe)
📈 Excess (Hypervitaminosis D)
Rare but possible with supplements
Hypercalcemia → nausea, vomiting, weakness, kidney stones, soft tissue calcification
6. Manifestations of Vitamin D Deficiency
🦴 5. Functions of Vitamin D
Vitamin D is indispensable for maintaining bone integrity, immune health, and overall physiological balance.
Vitamin D is essential for maintaining bone health and preventing hypocalcemic complications.
🧪 1. Chemistry & Forms
⚙️ 4. Metabolism & Activation
🎯 Key Takeaways
12. fat-soluble seco-steroid
Natural Sources:
Sunlight exposure: UV-B rays convert 7-dehydrocholesterol in the skin to Vitamin D₃.
Fatty fish (salmon, mackerel, tuna).
Egg yolks.
Fortified Foods:
Dairy products.
Breakfast cereals.
Orange juice.
Supplements:
Vitamin D₂ (ergocalciferol) and Vitamin D₃ (cholecalciferol).
10. Diagnostic Tests for Vitamin D Deficiency
2. Vitamin D: Requirements
3. Sources of Vitamin D
Fat-soluble secosteroid
Exists in two major forms:
Vitamin D₂ (Ergocalciferol): From plant sources and fortified foods
Vitamin D₃ (Cholecalciferol): Synthesized in skin (from 7-dehydrocholesterol under UV-B light) and from animal sources
Key Point: D₂ and D₃ are inactive and require two hydroxylation steps to become active.
Vitamin D deficiency affects calcium and phosphate homeostasis, leading to various clinical symptoms and conditions. Here are the key manifestations:
1. Musculoskeletal Issues
Rickets (in children):
Soft and weak bones.
Bowed legs or other skeletal deformities.
Stunted growth.
Osteomalacia (in adults):
Bone pain and tenderness.
Increased risk of fractures.
Osteoporosis:
Reduced bone density and structural integrity.
Susceptibility to fractures, especially in the elderly.
2. Hypocalcemia
Muscle cramps, spasms (tetany), or numbness.
Tingling in fingers or around the mouth.
Seizures in severe cases.
3. Immune Dysfunction
Increased risk of infections, including respiratory tract infections.
Poor wound healing and tissue repair.
4. Fatigue and Mood Disorders
Chronic fatigue.
Depression or mood swings, possibly due to altered neurochemical regulation.
5. Risk of Chronic Diseases
Higher susceptibility to:
Cardiovascular diseases.
Type 2 diabetes.
Autoimmune diseases.
Certain cancers (e.g., colorectal cancer).
1. Regulation of Calcium and Phosphate Levels
Promotes the absorption of calcium and phosphate from the intestine.
Maintains serum calcium levels, ensuring proper bone mineralization.
Prevents hypocalcemia by mobilizing calcium from bones when dietary intake is insufficient.
2. Bone Health
Supports normal bone growth and remodeling.
Prevents bone-related diseases like rickets in children and osteomalacia in adults.
Reduces the risk of fractures by maintaining bone density.
3. Immune System Modulation
Enhances immune defense by promoting the activity of T-cells and macrophages.
Plays a protective role against autoimmune disorders.
4. Muscle Function
Contributes to muscle strength and function by regulating calcium levels within muscle cells.
5. Cardiovascular Health
Linked to maintaining healthy blood pressure and reducing the risk of cardiovascular diseases.
May have anti-inflammatory effects on vascular tissues.
6. Role in Disease Prevention
Studies suggest a role in reducing the risks of diabetes, certain cancers (like colorectal cancer), and neurodegenerative disorders.
Skin: UV-B → 7-dehydrocholesterol → Vitamin D₃
Liver: Vitamin D → 25-hydroxylase → 25-hydroxyvitamin D [25(OH)D]
Major circulating form; measured for clinical assessment
Kidney: 25(OH)D → 1α-hydroxylase → 1,25-dihydroxyvitamin D [1,25(OH)₂D, Calcitriol]
Active hormone form
Enzyme stimulated by PTH, hypocalcemia, and hypophosphatemia
🧪 8. Clinical Aspects
Serum 25(OH)D:
Deficiency: <20 ng/mL
Insufficiency: 20–30 ng/mL
Sufficient: >30 ng/mL
👉(Always measure 25(OH)D, not calcitriol, for assessment.)
🧪 7. Normal Values
1️⃣ Fat-Soluble
Meaning: Soluble in lipids (fats), not in water.
Implications:
Absorbed in the intestine along with dietary fat.
Requires bile salts and micelles for absorption.
Stored in the liver and adipose tissue (so deficiency takes time to develop).
Risk of toxicity if taken in excess, unlike water-soluble vitamins, which are easily excreted.
Examples: Vitamins A, D, E, and K.
2️⃣ Secosteroid
Steroid: A Class of lipophilic molecules with a four-ring structure (cyclopentanoperhydrophenanthrene nucleus).
Seco- (Latin: “to cut”): Refers to a “broken” or “split” ring in the steroid structure.
👉 So, a secosteroid is a steroid molecule in which one of the four rings is broken.
In Vitamin D, the B-ring of the steroid nucleus is broken open by UV-B light in the skin, forming cholecalciferol (Vitamin D₃).
This unique structure makes Vitamin D biologically distinct from other steroids.
3️⃣ Why is Vitamin D important?
Being fat-soluble → requires proper fat absorption mechanisms.
Being a secosteroid → explains its prohormone nature and how UV-B light transforms 7-dehydrocholesterol into Vitamin D₃.
Its steroid-like structure explains why Vitamin D, once activated (Calcitriol), acts like a hormone by binding to nuclear receptors (Vitamin D Receptor, VDR) and regulating gene expression.
✅ In short:
A fat-soluble secosteroid means Vitamin D is a lipid-soluble vitamin that has a modified steroid structure with a broken ring, allowing it to act as a hormone in calcium-phosphate regulation and beyond.
9. Clinical Correlations
Chronic Kidney Disease (CKD): Low calcitriol due to ↓ 1α-hydroxylase → renal osteodystrophy
Hypoparathyroidism: Low PTH → ↓ calcitriol → hypocalcemia
Granulomatous disease (e.g., sarcoidosis, TB): Macrophages express 1α-hydroxylase → ↑ calcitriol → hypercalcemia
Vitamin D is a prohormone, not just a vitamin.
Activated in two steps: liver (25-hydroxy) → kidney (1,25-dihydroxy).
Maintains Ca²⁺-phosphate balance: absorption, bone, and kidney.
Deficiency → Rickets, Osteomalacia, Hypocalcemia.
Excess → Hypercalcemia, renal stones, calcification.
Clinically assessed via serum 25(OH)D levels.
25-Hydroxyvitamin D Test (25[OH]D):
Gold Standard Test for assessing Vitamin D levels.
Interpretation of Results:
Sufficient: >30 ng/mL (75 nmol/L).
Insufficient: 21-29 ng/mL (50-74 nmol/L).
Deficient: <20 ng/mL (50 nmol/L).
Reflects overall Vitamin D stores from diet, supplements, and sunlight.
Bone Mineral Density Test (BMD):
Detects bone weakness or early signs of osteoporosis in severe Vitamin D deficiency.
Performed via dual-energy X-ray absorptiometry (DEXA).
Calcium and Phosphate Levels:
Assesses serum calcium and phosphate to identify complications like hypocalcemia or hyperparathyroidism linked to Vitamin D deficiency.
Parathyroid Hormone (PTH) Test:
Elevated PTH may indicate secondary hyperparathyroidism due to Vitamin D deficiency.
Alkaline Phosphatase (ALP):
Higher levels may indicate bone turnover or damage.
11. Regulation
PTH: ↑ 1α-hydroxylase → ↑ Calcitriol
FGF-23: ↓ 1α-hydroxylase → ↓ Calcitriol (phosphate regulator)
Negative feedback: Calcitriol inhibits its own synthesis
12. Treatment Options for Vitamin D Deficiency
Lifestyle Adjustments:
Sunlight Exposure:
Spend 10-15 minutes in sunlight (without sunscreen) 2-3 times per week.
Focus on face, arms, and legs for optimal Vitamin D synthesis.
Increase dietary sources of Vitamin D (e.g., fatty fish, fortified dairy).
Vitamin D Supplementation:
Cholecalciferol (Vitamin D₃) or Ergocalciferol (Vitamin D₂):
Mild Deficiency: 800-2000 IU/day.
Severe Deficiency: 50,000 IU/week (prescription dose) for 8-12 weeks.
Maintenance doses prevent recurrence.
Calcium Supplementation:
Co-administer calcium supplements (500-1200 mg/day) with Vitamin D to support bone health.
Treatment of Underlying Causes:
Address malabsorption conditions (e.g., celiac disease, Crohn’s disease).
Manage medications interfering with Vitamin D metabolism (e.g., anticonvulsants, glucocorticoids).
Monitor Progress:
Repeat 25[OH]D testing after 3-6 months to ensure levels normalize.
Recommended Daily Intake:
Adults: 600-800 IU/day.
Elderly: 800-1000 IU/day.
Higher intake may be needed in regions with low sunlight exposure or in individuals with risk factors for deficiency.
Fat-Soluble Vitamin E
Vitamin E is a fat-soluble vitamin primarily recognized for its antioxidant properties, which protect cells from oxidative damage. It plays a crucial role in maintaining skin health, immune function, and cardiovascular integrity.
1. Daily Requirements of Vitamin E

Vitamin E’s Role in Cardiovascular Health
2. Dietary Sources of Vitamin E
Hypervitaminosis E (Vitamin E Toxicity)
Clinical Applications of Vitamin E in Heart Disease Prevention
8. Vitamin E: Its Role as an Antioxidant
3. Functions of Vitamin E
4. Clinical Aspects of Vitamin E
Vitamin E has diverse physiological roles, including:
Antioxidant Defense – Prevents lipid peroxidation, protecting cell membranes from oxidative stress.
Immune Enhancement – Strengthens immune response, reducing susceptibility to infections.
Skin Health – Maintains skin integrity by neutralizing free radicals, promoting wound healing.
Neurological Protection – Supports nerve function, preventing neurodegenerative disorders.
Cardiovascular Health – Reduces oxidation of LDL cholesterol, lowering the risk of atherosclerosis.
Hormonal Balance – Influences reproductive health and supports fertility.
Deficiency is rare but can lead to serious health issues, especially in individuals with fat malabsorption disorders or genetic conditions affecting vitamin E metabolism.
Risk factors include malabsorption syndromes (Crohn’s disease, cystic fibrosis), premature birth, and liver disease.
5. Vitamin E Deficiency: Manifestations
Vitamin E is used in various medical treatments:
Skin Disorders – Applied in topical creams for wound healing and reducing scars.
Neurological Conditions – May slow the progression of neurodegenerative diseases like Alzheimer’s.
Cardiovascular Support – Helps prevent oxidative stress-related heart diseases.
Reproductive Health – Supports fertility in both men and women by reducing oxidative stress.
6. Regulation of Vitamin E
7. Biological Importance of Vitamin E
Vitamin E is regulated via:
Absorption & Transport
Absorbed in the small intestine with dietary fats.
Transported via chylomicrons in the bloodstream to tissues.
Stored in the liver and adipose tissue for later use.
Excretion & Feedback Mechanism.
Excess vitamin E is excreted via bile and feces, preventing toxicity.
High doses may interfere with vitamin K absorption, affecting blood clotting
Vitamin E plays an indispensable role in cellular protection and systemic health:
Preserves cell membrane integrity by preventing oxidative damage.
Supports cardiovascular function by reducing LDL cholesterol oxidation
Enhances immune resilience to combat infections effectively.
Promotes neuroprotection, delaying cognitive decline in aging.
Influences reproductive health, ensuring fertility and fetal development.
How Vitamin E Acts as an Antioxidant
Vitamin E prevents the oxidation of lipids in cell membranes, maintaining their structural integrity.
It works by:
Scavenging free radicals – Donates electrons to stabilize highly reactive free radicals.
Preventing lipid peroxidation – Stops oxidative damage to polyunsaturated fatty acids in cell membranes.
Protecting proteins and DNA – Shields essential biomolecules from oxidative stress.
Enhancing other antioxidants – Works synergistically with vitamin C, glutathione, and selenium to maintain redox balance.
Vitamin E is one of the body's most powerful fat-soluble antioxidants, protecting cells and tissues from oxidative damage. It neutralizes harmful molecules called free radicals, which are produced during metabolism and by environmental stressors such as pollution, UV radiation, and toxins.
1. Immune System Support
Strengthens the immune response by protecting immune cells from oxidative damage.
Enhances T-cell function, improving defense against infections.
Reduces inflammation by modulating immune pathways.
2. Skin and Wound Healing
Promotes skin regeneration and reduces scarring.
Used in topical treatments to alleviate burns, wounds, and dermatitis.
Helps in moisturizing dry and damaged skin.
3. Cardiovascular Protection
Prevents oxidation of LDL cholesterol, reducing the risk of atherosclerosis.
Improves blood vessel health and reduces blood clot formation.
It may help regulate blood pressure through vascular relaxation.
4. Neurological Health
Protects neurons from oxidative stress, potentially preventing neurodegenerative diseases like Alzheimer's.
Supports proper nerve conduction and muscle coordination.
It may help reduce symptoms in Parkinson’s disease.
5. Hormonal and Reproductive Health
Plays a role in fertility by protecting sperm and egg cells from oxidative damage.
Supports reproductive health by regulating hormonal balance.
May help alleviate menstrual pain and menopausal symptoms.
6. Eye Health
Prevents oxidative damage in the retina, reducing the risk of macular degeneration.
Protects against cataract formation by stabilizing lens proteins.
7. Anti-Inflammatory Effects
Helps in reducing inflammation linked to chronic diseases.
Beneficial in conditions like arthritis and inflammatory bowel diseases.
A. Cellular Protection
Prevents premature cell aging and degenerative changes.
Reduces oxidative stress that contributes to cancer, neurodegenerative diseases, and cardiovascular disorders.
B. Skin Health
Neutralizes UV-induced oxidative damage, reducing wrinkles, pigmentation, and inflammation.
Helps in wound healing by supporting collagen formation.
C. Cardiovascular Benefits
Inhibits LDL oxidation, preventing plaque formation in arteries.
Reduces atherosclerosis risk, improving heart health.
D. Neurological Protection
Protects neurons from oxidative damage, reducing risks of Alzheimer’s disease and Parkinson’s disease.
E. Immune Function
Strengthens immune responses by reducing oxidative damage to immune cells.
👉 Consuming vitamin E-rich foods ensures adequate antioxidant defense:
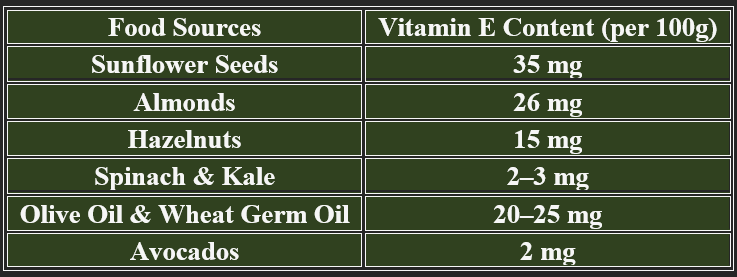
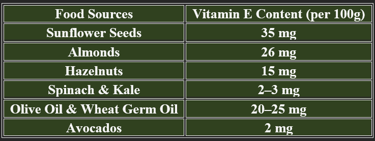
Almonds, sunflower seeds, spinach, olive oil, avocado, nuts
Wheat germ, pumpkin seeds, fish, fortified cereals
👉Vitamin E is essential for cellular integrity, cardiovascular protection, neuroprotection, and immune health. Neutralizing free radicals prevents oxidative damage that leads to chronic diseases.


9. Biological Importance of Vitamin E as an Antioxidant
Vitamin E is far more than just an antioxidant—it plays several important physiological roles that contribute to overall health and well-being. Here are its other key functions beyond antioxidant protection:
Vitamin E is crucial not just as an antioxidant but for immune function, cardiovascular health, neurological protection, skin repair, reproductive health, and anti-inflammatory support. Ensuring adequate intake helps maintain cellular integrity and overall physiological balance.
Plasma Concentration of Vitamin E
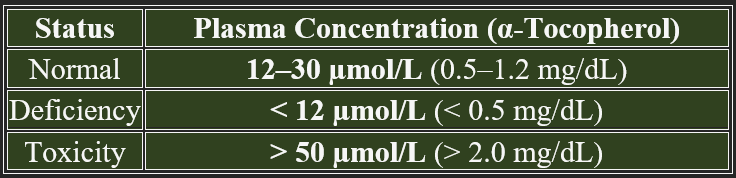

Vitamin E levels in the blood help assess an individual's nutritional status, antioxidant capacity, and potential deficiency or toxicity. The normal plasma concentration of vitamin E is primarily measured as α-tocopherol, the most biologically active form.
The typical plasma range varies based on dietary intake, absorption efficiency, and physiological demand.
Note: Plasma levels below 12 µmol/L indicate a deficiency, while levels above 50 µmol/L may suggest excessive supplementation.
1. Factors Influencing Plasma Vitamin E Levels
Vitamin E concentration is regulated by several factors:
Dietary Intake – Levels depend on vitamin E-rich food
consumption (nuts, oils, seeds).
Lipid Concentration – Vitamin E is transported by lipoproteins,
making plasma levels closely linked to lipid metabolism.
Absorption & Liver Function – Disorders affecting fat absorption (e.g., cholestasis, cystic fibrosis) can lower vitamin E levels.
Oxidative Stress & Inflammation – Increased antioxidant demand in diseases can deplete vitamin E reserves.
2. Clinical Significance of Plasma Vitamin E Measurement
Monitoring plasma vitamin E levels helps evaluate nutritional adequacy, deficiency risks, and therapeutic needs. Maintaining optimal levels through balanced dietary intake and proper lipid metabolism is essential for overall health.
Deficiency Diagnosis – Used to assess risks for neuropathy, hemolytic anemia, and retinal degeneration.
Therapeutic Monitoring – Helps optimize supplementation in conditions requiring high antioxidant support (e.g., neurodegenerative disorders).
Toxicity Assessment – Excessive vitamin E intake can impair blood clotting and interfere with vitamin K metabolism.
Vitamin E’s Interactions with Other Nutrients
Hypervitaminosis E refers to excessive vitamin E intake, leading to bleeding disorders, impaired immune function, and potential interactions with other vitamins. Since vitamin E is fat-soluble, it can accumulate in tissues and cause adverse effects.
Excess vitamin E interferes with platelet aggregation, prolonging bleeding times and increasing hemorrhage risks, especially in individuals on anticoagulant therapy. Hypervitaminosis E impairs blood clotting, weakens muscle function, and lowers immune resilience. Careful regulation of supplement intake prevents toxicity-related disorders, ensuring balanced health.


A. Vitamin C (Synergistic Antioxidant Action)
Vitamin E neutralizes lipid peroxidation, preventing oxidative damage to cell membranes.
Vitamin C regenerates oxidized vitamin E, ensuring continuous antioxidant protection.
Deficiency in one affects the other, increasing oxidative stress.
B. Vitamin K (Impact on Blood Clotting)
Excess vitamin E antagonizes vitamin K, increasing bleeding risks by impairing clot formation.
This interaction is critical for patients on anticoagulants (e.g., warfarin).
C. Selenium (Enhances Antioxidant Function)
Glutathione peroxidase (selenium-dependent enzyme) complements vitamin E, reducing lipid oxidation.
Deficiency of selenium may reduce vitamin E effectiveness.
D. Polyunsaturated Fatty Acids (PUFAs) and Vitamin E
PUFAs increase oxidative stress, raising the need for vitamin E protection.
Diets high in omega-3 fatty acids require higher vitamin E intake.
A. Prevention of LDL Oxidation
Vitamin E reduces oxidation of LDL (bad cholesterol), preventing plaque buildup.
Oxidized LDL contributes to atherosclerosis, leading to heart attacks.
B. Blood Vessel Protection
Maintains endothelial integrity, preventing damage to arterial walls.
Supports nitric oxide production, improving blood flow and reducing hypertension.
C. Anti-Inflammatory Effects
Reduces C-reactive protein (CRP), lowering inflammation in arteries.
Prevents foam cell formation, decreasing plaque accumulation.
D. Stroke and Heart Disease Risk Reduction
Improves circulation and prevents excessive clot formation.
May lower stroke risk, but excessive intake increases bleeding tendency.
3. Vitamin E Supplementation and Safety
✔ Supplementation benefits individuals with low dietary intake or specific heart conditions.
✔ Recommended upper limit: 1,000 mg/day; exceeding this increases bleeding risk.
❌ Avoid excessive supplementation in patients on anticoagulants (Warfarin, Aspirin).
Conclusion
Vitamin E plays a critical role in heart disease prevention, supporting antioxidant defense, vascular protection, and anti-inflammatory activity. A balanced intake from natural sources ensures cardiovascular benefits while avoiding excess risks.
A. Prevention of LDL Oxidation
Vitamin E reduces oxidation of LDL (bad cholesterol), lowering the risk of atherosclerosis.
Oxidized LDL contributes to arterial plaque formation, leading to heart disease.
B. Blood Vessel Protection
Maintains vascular integrity, preventing endothelial damage.
Supports nitric oxide production, improving blood flow and reducing hypertension risks.
C. Anti-Inflammatory Effects
Reduces C-reactive protein (CRP) levels, lowering systemic inflammation in arteries.
Prevents foam cell formation, decreasing plaque buildup.
D. Stroke and Heart Disease Prevention
Improves circulation and prevents clot formation in arteries.
Studies suggest optimal vitamin E intake lowers stroke risk, but excess intake may increase bleeding tendency.
Conclusion
Vitamin E interacts with vitamin C, K, selenium, and PUFAs, while playing a major role in cardiovascular protection by preventing LDL oxidation, inflammation, and arterial damage. Proper intake is crucial for heart health, but excessive intake can increase the risk of bleeding.
Vitamin E: Biosynthesis, Absorption, Transport, and Storage
Vitamin E is a fat-soluble antioxidant essential for cell protection, immune function, and neurological health. It exists in eight forms, with α-tocopherol being the most biologically active.
1. Biosynthesis of Vitamin E
Vitamin E is naturally synthesized only by plants and some cyanobacteria, meaning humans must obtain it through diet.
A. Plant Biosynthesis Process
Synthesized via the terpenoid pathway, using isoprenoid precursors.
Forms include tocopherols and tocotrienols, depending on the enzyme action.
α-Tocopherol is the primary form in humans due to its specific transport proteins.
B. Sources of Vitamin E
✔ Nuts & Seeds – Almonds, sunflower seeds, hazelnuts.
✔ Vegetable Oils – Olive oil, soybean oil, wheat germ oil.
✔ Leafy Greens – Spinach, kale, broccoli.
2. Absorption of Vitamin E
Since vitamin E is fat-soluble, its absorption depends on lipid digestion and bile salts.
A. Intestinal Absorption
Absorbed primarily in the duodenum and jejunum.
Requires bile salts and pancreatic lipase for emulsification.
Taken up by enterocytes, where it is incorporated into chylomicrons for transport.
B. Factors Enhancing Absorption
✔ Dietary fat intake improves solubility.
✔ Healthy liver function ensures bile production.
C. Factors Reducing Absorption
❌ Fat malabsorption disorders (e.g., Crohn’s disease, cystic fibrosis).
❌ Low bile acid production (liver disease, gallbladder dysfunction).
3. Transport of Vitamin E
After absorption, vitamin E is transported through lipoprotein pathways.
A. Chylomicron Transport
Enterocytes package vitamin E into chylomicrons, which enter the lymphatic system.
Delivered first to the liver, where it is processed for systemic distribution.
B. Hepatic Processing
The liver recognizes α-tocopherol and incorporates it into lipoproteins (LDL, VLDL, HDL).
Tocopherol Transfer Protein (TTP) ensures preferential transport of α-tocopherol.
C. Circulatory Transport
Vitamin E moves via lipoproteins, reaching target cells through receptor-mediated uptake.
4. Storage of Vitamin E
Vitamin E is not stored in large quantities but is maintained in fat tissues and lipoproteins.
A. Primary Storage Sites
✔ Adipose tissue – Major long-term storage.
✔ Liver – Temporarily stores vitamin E before redistribution.
✔ Muscle and Brain – Low amounts for neurological function.
B. Regulation of Release
✔ Gradual release from adipose tissue into circulation as needed.
✔ Excess excretion via bile and feces to prevent toxicity.
Fat-Soluble Vitamin K
Vitamin K is a fat-soluble vitamin essential for blood clotting, bone metabolism, and cardiovascular health.
K₁ (Phylloquinone): from green leafy vegetables.
K₂ (Menaquinone): produced by intestinal bacteria.
K₃ (Menadione): synthetic, water-soluble form.
Rich sources: spinach, kale, broccoli, cabbage, liver, eggs.
Body stores are limited; regular intake is needed.
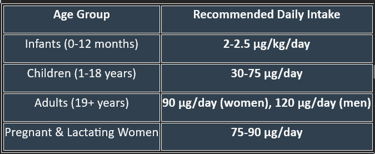
2. Daily Requirements of Vitamin K
1. The main natural forms and dietary sources
Vitamin K is a fat-soluble vitamin essential for blood coagulation and bone health, and it plays a crucial role in several physiological processes. Its major functions can be grouped into two areas:
1. Role in Blood Coagulation (Hemostasis)
Vitamin K acts as a coenzyme for γ-carboxylation of glutamic acid residues in certain proteins.
This modification is required for binding calcium ions (Ca²⁺), which is essential for the clotting cascade function.
It is needed for the activation of clotting factors:
Factor II (Prothrombin)
Factor VII
Factor IX
Factor X
Also required for two natural anticoagulants:
Protein C
Protein S
👉 Without Vitamin K → bleeding disorders, prolonged prothrombin time (PT).
2. Role in Bone Metabolism
Vitamin K is essential for γ-carboxylation of osteocalcin, a protein secreted by osteoblasts.
This allows osteocalcin to bind calcium, improving bone mineralization.
Deficiency may contribute to osteoporosis and fractures.
3. Role in Vascular Health
Vitamin K–K-dependent Matrix Gla protein (MGP) prevents vascular calcification, maintaining vessel elasticity.
Functions of Vitamin K
Clinical Aspects of Vitamin K
Vitamin K is used in various medical treatments:
Warfarin Antidote – Administered to counteract excessive anticoagulation from warfarin therapy.
Hemorrhagic Disease Prevention – Newborns receive vitamin K injections to prevent bleeding disorders.
Bone Health Support – May be used to reduce osteoporosis risk in elderly individuals.
Cardiovascular Disease Management – Helps prevent arterial calcification, reducing heart disease risks.
Newborns: At high risk of deficiency because vitamin K does not cross the placenta well, breast milk is low in K, and gut flora is not yet established. This is why vitamin K injection is routinely given at birth.
Drug interactions: Long-term use of broad-spectrum antibiotics (gut flora suppression) or warfarin (antagonist of the vitamin K cycle) can lead to deficiency.
Monitoring: Prothrombin time (PT/INR) is a key clinical test sensitive to vitamin K status.
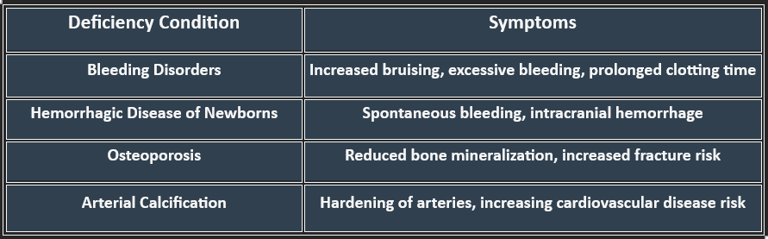
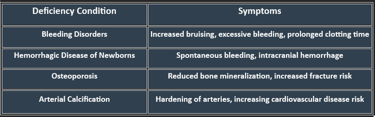
Vitamin K Deficiency: Manifestations
Deficiency can lead to serious complications, particularly affecting coagulation and bone integrity. Risk factors include malabsorption syndromes (Crohn’s disease, cystic fibrosis), liver disorders, and long-term antibiotic use.
Causes:
Poor dietary intake (rare, except in malnutrition).
Newborn state (physiological deficiency).
Fat malabsorption (biliary obstruction, pancreatic insufficiency).
Prolonged antibiotic therapy.
Warfarin toxicity (drug-induced functional deficiency).
Manifestations:
Bleeding tendency: easy bruising, mucosal bleeding, hematuria, GI bleed.
Prolonged prothrombin time (PT).
In neonates: Hemorrhagic disease of the newborn.
Regulation of Vitamin K
Vitamin K levels are regulated through:
✅Absorption & Transport
Vitamin K1 is absorbed in the small intestine with dietary fats.
Vitamin K2 is synthesized by gut bacteria in the colon and absorbed in the bloodstream.
Transported via lipoproteins and stored in the liver.
✅Metabolism & Feedback Mechanism
Recycling via the Vitamin K Cycle – Allows continuous activation of clotting factors.
Excretion – Excess vitamin K is eliminated via bile and feces.
✅ Regulation
Maintained by dietary intake + gut microbial synthesis.
Vitamin K undergoes a recycling process (vitamin K cycle) in the liver:
Reduced vitamin K (hydroquinone form) acts as the cofactor.
After γ-carboxylation, it becomes oxidized (epoxide form).
Vitamin K epoxide reductase (VKORC1) regenerates the active reduced form.
Warfarin inhibits VKORC1, blocking recycling and thereby inhibiting clotting factor activation.
Hypervitaminosis K (Vitamin K Toxicity)
Hypervitaminosis K refers to excessive vitamin K intake, leading to blood clotting abnormalities, vascular complications, and potential liver toxicity. While vitamin K is crucial for coagulation and bone metabolism, excessive intake—especially from synthetic forms—can pose risks.
1. Causes of Hypervitaminosis K
Excessive Supplementation – Overconsumption of vitamin K-rich foods or high-dose synthetic supplements.
Medical Overuse – Incorrect administration in anticoagulant therapy reversal.
Neonatal Vitamin K Overdose – High doses in newborns, leading to hemolysis or jaundice.
2. Symptoms of Hypervitaminosis K
Long-term excess vitamin K may disrupt normal anticoagulation, leading to vascular complications.
Biological Importance:
Essential for hemostasis (prevents uncontrolled bleeding).
Maintains bone strength and reduces the risk of osteoporosis.
Prevents pathological calcification of arteries and soft tissues.
Supports both coagulation and anticoagulation balance (via proteins C and S).

BLOG
Join us to explore medical biochemistry intricacies.
WRITE TO US
© 2024. All rights reserved.
