Buffer Systems: Guardians of Human Body pH
CHEMICAL BASIS OF LIFEMEDICAL BIOCHEMISTRY
9/15/20252 min read
The Silent Guardians of Our pH
When we think of health, most of us imagine food, exercise, or even our heartbeat. But hidden behind all of these is something quieter yet vital — the balance of acids and bases in our body. This balance is measured by pH, and keeping it within a narrow window (around 7.35–7.45 in blood) is critical for life. Even a small shift can disrupt how our enzymes work, how oxygen is carried, and how our organs function.
So, how does the body achieve this delicate balance?
The unsung heroes here are the buffer systems.
What is a Buffer?
A buffer is like a shock absorber in a car — it minimizes sudden jolts.
Chemically, a buffer is a mixture of a weak acid and its conjugate base (or vice versa) that resists sudden changes in pH when acids or bases are added.
In the human body, buffers step in whenever there’s a threat of “too much acid” (acidosis) or “too much base” (alkalosis).
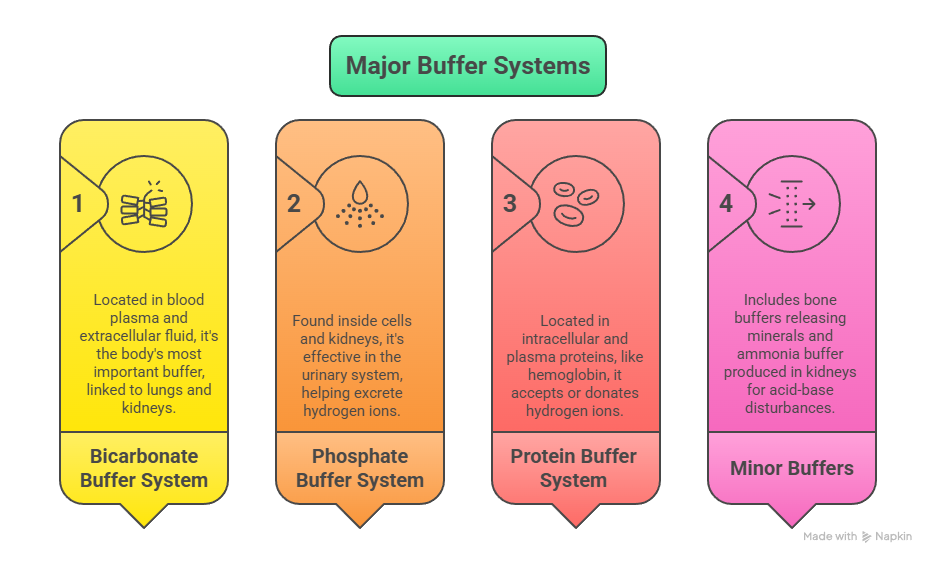
Major Buffer Systems in the Human Body
1. Bicarbonate Buffer System
Location: Blood plasma and extracellular fluid
How it works:
Carbonic acid (H₂CO₃) ↔ Bicarbonate (HCO₃⁻) + H⁺Why it matters:
This is the body’s most important buffer, especially in the blood. It is directly linked to the lungs (through CO₂ exhalation) and kidneys (through bicarbonate reabsorption), giving it a dynamic role.
👉 Clinical note: Disturbances here cause respiratory acidosis/alkalosis or metabolic acidosis/alkalosis — common in lung disease, kidney disorders, or uncontrolled diabetes.
2. Phosphate Buffer System
Location: Inside cells and in the kidneys
How it works:
Dihydrogen phosphate (H₂PO₄⁻) ↔ Mono hydrogen phosphate (HPO₄²⁻) + H⁺Why it matters:
Particularly effective in the urinary system, where it helps excrete hydrogen ions, maintaining acid-base balance.
👉 Clinical note: Plays a role in conditions like renal tubular acidosis.
3. Protein Buffer System
Location: Both intracellular and plasma proteins
How it works:
Proteins (like hemoglobin in red blood cells) can accept or donate hydrogen ions thanks to their amino acid side chains.Why it matters:
Hemoglobin, in particular, is a powerful buffer that helps maintain blood pH while transporting oxygen and carbon dioxide.
👉 Clinical note: Hemoglobin’s buffering role becomes crucial in conditions like chronic obstructive pulmonary disease (COPD), where CO₂ retention challenges the pH balance.
4. Minor Buffers
Bone buffers: During chronic acidosis, bone minerals release carbonate and phosphate to neutralize excess acid — but at the cost of bone strength.
Ammonia buffer (NH₃/NH₄⁺): Produced in the kidneys, especially helpful in long-term acid-base disturbances.
Why Does It Matter?
Think of buffer systems as your body’s “safety net.” Without them, everyday processes like digestion, exercise, or even breathing could cause dangerous pH swings.
For the common reader: Buffers are why your body doesn’t go into crisis every time you eat a spicy meal, hold your breath, or have a stressful day.
For the medical student: Understanding buffers is the foundation for interpreting arterial blood gases (ABGs), managing ICU patients, and diagnosing acid-base disorders.
🚑 Clinical Significance at a Glance
Acidosis (pH < 7.35): Seen in uncontrolled diabetes (ketoacidosis), kidney failure, or severe diarrhea.
Alkalosis (pH > 7.45): Seen in prolonged vomiting, hyperventilation, or excessive antacid use.
Buffer systems, along with the lungs and kidneys, act quickly or slowly to bring pH back to normal.
✨ Takeaway
Buffer systems may not make headlines like the heart or brain, but they are just as vital. They are the quiet custodians that keep our internal environment steady, ensuring that life’s chemistry runs smoothly. Whether you’re a curious reader or a budding medical professional, appreciating these systems is like discovering the silent guardians working tirelessly within you.
BLOG
Join us to explore medical biochemistry intricacies.
WRITE TO US
© 2024. All rights reserved.
